Summary
Students engage in hands-on activities that provide an opportunity to learn about biological and environmental engineering. Students learn about perfluoroalkyl and polyfluoroalkyl substances (PFAS), and how to remove them from drinking water. Students are given water from a local water source and must create a filtration system. The students will then take the filtered water and attempt to purify that water.
Engineering Connection
Chemical engineers play a vital role in providing clean water by designing and improving processes that treat and purify water. They develop filtration systems using materials such as activated charcoal and zeolites, apply chemical treatments such as chlorine to disinfect, and design advanced systems to remove harmful chemicals such as PFAS. They are also involved in desalination, turning seawater into drinkable water, and wastewater treatment, ensuring safe reuse or release of treated water. Additionally, chemical engineers focus on reducing water pollution, conserving resources, and creating sustainable water management practices to ensure clean water for future generations.
Learning Objectives
After this activity, students should be able to:
- Describe what PFAS are, where they come from, and why they are harmful to the environment and human health.
- Design and construct a simple water filtration system using readily available materials.
- Apply problem-solving and critical thinking skills to improve their water filtration systems based on test results.
- Practice communicating their ideas and collaborating with peers to achieve a common goal.
Educational Standards
Each TeachEngineering lesson or activity is correlated to one or more K-12 science,
technology, engineering or math (STEM) educational standards.
All 100,000+ K-12 STEM standards covered in TeachEngineering are collected, maintained and packaged by the Achievement Standards Network (ASN),
a project of D2L (www.achievementstandards.org).
In the ASN, standards are hierarchically structured: first by source; e.g., by state; within source by type; e.g., science or mathematics;
within type by subtype, then by grade, etc.
Each TeachEngineering lesson or activity is correlated to one or more K-12 science, technology, engineering or math (STEM) educational standards.
All 100,000+ K-12 STEM standards covered in TeachEngineering are collected, maintained and packaged by the Achievement Standards Network (ASN), a project of D2L (www.achievementstandards.org).
In the ASN, standards are hierarchically structured: first by source; e.g., by state; within source by type; e.g., science or mathematics; within type by subtype, then by grade, etc.
NGSS: Next Generation Science Standards - Science
| NGSS Performance Expectation | ||
|---|---|---|
|
MS-ESS3-3. Apply scientific principles to design a method for monitoring and minimizing a human impact on the environment. (Grades 6 - 8) Do you agree with this alignment? |
||
| Click to view other curriculum aligned to this Performance Expectation | ||
| This activity focuses on the following Three Dimensional Learning aspects of NGSS: | ||
| Science & Engineering Practices | Disciplinary Core Ideas | Crosscutting Concepts |
| Apply scientific principles to design an object, tool, process or system. Alignment agreement: | Human activities have significantly altered the biosphere, sometimes damaging or destroying natural habitats and causing the extinction of other species. But changes to Earth's environments can have different impacts (negative and positive) for different living things. Alignment agreement: | Relationships can be classified as causal or correlational, and correlation does not necessarily imply causation. Alignment agreement: The uses of technologies and any limitations on their use are driven by individual or societal needs, desires, and values; by the findings of scientific research; and by differences in such factors as climate, natural resources, and economic conditions. Thus technology use varies from region to region and over time.Alignment agreement: |
| NGSS Performance Expectation | ||
|---|---|---|
|
MS-ETS1-1. Define the criteria and constraints of a design problem with sufficient precision to ensure a successful solution, taking into account relevant scientific principles and potential impacts on people and the natural environment that may limit possible solutions. (Grades 6 - 8) Do you agree with this alignment? |
||
| Click to view other curriculum aligned to this Performance Expectation | ||
| This activity focuses on the following Three Dimensional Learning aspects of NGSS: | ||
| Science & Engineering Practices | Disciplinary Core Ideas | Crosscutting Concepts |
| Define a design problem that can be solved through the development of an object, tool, process or system and includes multiple criteria and constraints, including scientific knowledge that may limit possible solutions. Alignment agreement: | The more precisely a design task's criteria and constraints can be defined, the more likely it is that the designed solution will be successful. Specification of constraints includes consideration of scientific principles and other relevant knowledge that is likely to limit possible solutions. Alignment agreement: | All human activity draws on natural resources and has both short and long-term consequences, positive as well as negative, for the health of people and the natural environment. Alignment agreement: The uses of technologies and any limitations on their use are driven by individual or societal needs, desires, and values; by the findings of scientific research; and by differences in such factors as climate, natural resources, and economic conditions.Alignment agreement: |
| NGSS Performance Expectation | ||
|---|---|---|
|
MS-PS1-3. Gather and make sense of information to describe that synthetic materials come from natural resources and impact society. (Grades 6 - 8) Do you agree with this alignment? |
||
| Click to view other curriculum aligned to this Performance Expectation | ||
| This activity focuses on the following Three Dimensional Learning aspects of NGSS: | ||
| Science & Engineering Practices | Disciplinary Core Ideas | Crosscutting Concepts |
| Gather, read, and synthesize information from multiple appropriate sources and assess the credibility, accuracy, and possible bias of each publication and methods used, and describe how they are supported or not supported by evidence. Alignment agreement: | Each pure substance has characteristic physical and chemical properties (for any bulk quantity under given conditions) that can be used to identify it. Alignment agreement: Substances react chemically in characteristic ways. In a chemical process, the atoms that make up the original substances are regrouped into different molecules, and these new substances have different properties from those of the reactants.Alignment agreement: | Structures can be designed to serve particular functions by taking into account properties of different materials, and how materials can be shaped and used. Alignment agreement: Engineering advances have led to important discoveries in virtually every field of science, and scientific discoveries have led to the development of entire industries and engineered systems.Alignment agreement: The uses of technologies and any limitations on their use are driven by individual or societal needs, desires, and values; by the findings of scientific research; and by differences in such factors as climate, natural resources, and economic conditions. Thus technology use varies from region to region and over time.Alignment agreement: |
Materials List
Day 1:
Each student needs:
- Access to map of PFAS found in the United States: https://www.ewg.org/areas-focus/toxic-chemicals/pfas-chemicals
- 1 Notice and Wonder Sheet
- 1 pen or pencil
For the class to share:
- 1 laptop/computer with project and internet access (to show video)
Day 2:
Each student needs:
- 1 copy of the article All the Stuff in Your Home That Might Contain PFAS ‘Forever Chemicals’
- 1 highlighter
- 1 Filtration System Design Packet
For the class to share:
- 1 laptop/computer with project and internet access (to show video)
Day 3:
Each group needs:
- 1 plastic 2-liter bottle with the base cut off
- 1 ruler
- 1 cup of dirty water from a local body of water
- 1 plastic cup (to collect filtered water)
For the class to share:
- coffee filters
- gravel (large and small)
- sand (smooth and coarse)
- marker (to label filtered water cups)
- (optional) carbonated charcoal for one group to use
Day 4:
Each group needs:
Day 5:
Each group needs:
- 1-2 cups of water (with 2-3 drops of food coloring)
- 1 plastic 2-liter bottle with the base cut off
- 2-3 clear plastic cups to collect the water samples
- activated charcoal (presoaking will take away its darker color)
- 4 cotton balls
- Filtration System Design Packet
For the class to share:
- water quality tester
- 1 laptop/computer with project and internet access (to show video)
- food coloring
Worksheets and Attachments
Visit [www.teachengineering.org/activities/view/uok-2949-safe-drinking-water-filtration-system-activity] to print or download.Pre-Req Knowledge
Students should:
- Be familiar with the engineering design process.
- Be able to analyze situations and think logically about solutions.
- Be able to work effectively in groups, share tasks, and collaborate on projects.
- Understand the importance of clean water and the impact of pollution.
- Know how to use a ruler to measure.
- Understand the process of filtration.
Introduction/Motivation
Imagine a super powerful chemical that’s used in a lot of things you touch and use every day. Perfluoroalkyl and polyfluoroalkyl substances, known as PFAS, are a group of chemicals that are really good at resisting water, oil, and heat. This makes them useful in all kinds of products, including non-stick pans, water-resistant jackets, and even the packaging for fast food!
What is the problem with PFAS? PFAS are special because they last a really long time and don’t break down easily. That means, even after we use these products, the chemicals can stick around in the environment, like in the water we drink, for years! Scientists have found PFAS in rivers, lakes, and even the ocean. Unfortunately, these chemicals can cause health problems for both people and animals.
PFAS can build up in our bodies over time and affect our health in several harmful ways. These chemicals are persistent, which means they don’t break down or go away easily, so they stay in our environment and in our bodies for a long time. Here’s how they can cause harm:
- Toxic to organs and systems: PFAS can affect different parts of the body, such as the liver, kidneys, and immune system. Studies have shown that long-term exposure to PFAS may lead to liver damage, kidney problems, and weakened immunity, making it harder for the body to fight off infections.
- Cancer risk: Some types of PFAS have been linked to an increased risk of certain cancers, such as kidney and testicular cancer. This happens because these chemicals can interfere with the way our cells work and grow, which might cause cancerous changes over time.
- Hormonal effects: PFAS can interfere with hormones in the body, including those related to growth, reproduction, and metabolism. This can lead to problems such as low birth weight in babies, developmental delays in children, and fertility issues in adults.
- Increased cholesterol levels: Some studies have shown that PFAS exposure can cause higher levels of cholesterol in the blood, which is a risk factor for heart disease.
- Environmental harm: When PFAS enter the environment, they can affect wildlife, too. Fish and animals can accumulate these chemicals in their bodies, which can harm their health and disrupt the ecosystem. This, in turn, can affect human health when we consume contaminated food.
Because PFAS don't break down easily, they can stick around in our environment and in our bodies, making them a long-term concern for our health. This is why scientists and engineers are studying ways to remove them from drinking water and other sources.
Today we are going to become chemical engineers and work to solve the problem of safe drinking water.
Procedure
Background
Perfluoroalkyl and polyfluoroalkyl substances (PFAS) are a group of human-made chemicals used in various industries for their water- and oil-resistant properties. They are often found in products such as nonstick cookware, water-repellent clothing, firefighting foam, and food packaging. PFAS are concerning because they are persistent in the environment and human body, earning them the nickname "forever chemicals." Long-term exposure to PFAS has been linked to adverse health effects, including cancer, liver damage, immune system disruption, and developmental issues. Their environmental persistence makes cleanup and regulation challenging, and they are a growing concern for public health and environmental safety.
Chemical engineering is a branch of engineering that focuses on the design, optimization, and operation of processes that convert raw materials into valuable products, typically involving chemical reactions. It combines principles from chemistry, physics, biology, mathematics, and economics to develop efficient, safe, and sustainable processes in industries such as manufacturing, energy, pharmaceuticals, food production, and environmental protection. Chemical engineers work on processes such as refining petroleum, creating plastics, and developing renewable energy technologies.
Before the Activity
Day 1:
- Ensure that all students have access to the map of PFAS found in the United States: https://www.ewg.org/areas-focus/toxic-chemicals/pfas-chemicals.
- Make copies of the Notice and Wonder Sheet (1 per student).
- Set up laptop/computer and projector to show the following Nightline video (8:31 minutes): https://www.youtube.com/embed/Tkkuil-U6qQ.
Day 2:
- Set up laptop/computer and projector to show the video “What Does a Chemical Engineer Do?” (6:23 minutes): https://www.youtube.com/watch?v=k-7B_YfHWXQ.
- Make copies of the article All the Stuff in Your Home That Might Contain PFAS ‘Forever Chemicals’ (1 per student).
- Make copies of the Filtration System Design Packet (1 per student).
Day 3:
- Collect dirty water from a local water source, enough for each group to have 3-4 cups to test.
- Gather activity materials.
- Make sure each group has a plastic bottle with the base cut off.
Day 4:
- Gather dirty water from a local body of water.
- Gather activity materials.
Day 5:
- Make copies of the Removal of PFAS Worksheet (1 per student).
- Gather activity materials.
- Optional: Presoak the activated charcoal to take away its darker color.
- Place 2-3 drops of food coloring into each water sample.
- Know how to use the water quality tester.
- Set up laptop/computer and projector to show the NBC News video “Engineers Develop Water Filtration System That Permanently Removes ‘Forever Chemicals’” (4:14 minutes): https://www.youtube.com/embed/ov7FTzDQy-k.
With the Students
Day 1: Ask: Introduction to PFAS and safe drinking water
- Read the Introduction / Motivation section to motivate the students.
- Display the map of PFAS contamination in the United States on the screen: https://www.ewg.org/areas-focus/toxic-chemicals/pfas-chemicals.
- Hand out one Notice and Wonder Sheet to each student.
- Prompt students: "Take a few minutes to look at this map, and jot down what you notice on the 'Notice' side of your sheet."
- Allow students time to talk with a shoulder partner about what they noticed on the map and what they wondered. Listen for observations related to concentrated areas of PFAS, particularly around military bases. This can lead to questions about why these areas are affected.
- Point out human activities (such as industrial use and military bases) as causes of PFAS contamination.
- Show this clip from Nightline (8:31 minutes): https://www.youtube.com/embed/Tkkuil-U6qQ.
- Have students fill out the “Wonder” side of the sheet with questions they still have.
- Go around the classroom and have each student share one new thing they learned from today’s activity.
Day 2: Research: Introduction to chemical engineering
- Introduce chemical engineering and chemical engineers:
- Chemical engineering: “Chemical engineering is a branch of engineering that focuses on the design, optimization, and operation of processes that convert raw materials into valuable products, typically involving chemical reactions. It combines principles from chemistry, physics, biology, mathematics, and economics to develop efficient, safe, and sustainable processes in industries such as manufacturing, energy, pharmaceuticals, food production, and environmental protection.”
- Chemical engineers: “Broadly, chemical engineers conceive and design processes involved in chemical manufacturing. The main role of chemical engineers is to design and troubleshoot processes to produce chemicals, fuels, foods, pharmaceuticals, and biologicals, to name just a few. They are most often employed by large-scale manufacturing plants to maximize productivity and product quality while minimizing costs.” (American Chemical Society Website)
- Show this video to give students an idea of what chemical engineers do (6:23 minutes): https://www.youtube.com/watch?v=k-7B_YfHWXQ.
- Explain the topic of the activity: "Today, we are going to explore ways chemical engineers separate mixtures. They are working on finding ways to separate PFAS, or 'forever chemicals,' from water to make drinking water safer.”
- Distribute this article for students to read: https://time.com/6281242/pfas-forever-chemicals-home-beauty-body-products.
- As students read the article, have them highlight key information that is new or surprising to them.
- Hand out one Filtration System Design Packet to each student.
- Bring the class back together and have a classroom discussion about how chemical engineers can separate liquids from solids, and explain why this is important for solving the PFAS problem.
- Give students five minutes to fill out the Research and Brainstorm section of the Filtration System Design Packet.
- Go around the classroom and have each student share one new thing they learned from the reading with the class.
Day 3: Brainstorm, Plan and Build
- Explain to the students: “Chemical engineers use a wide range of separation techniques to separate material. Yesterday, you read and started your research on learning about PFAS. Today we are going to look at how we can separate impurities from the water.”
- Discuss/review the steps of the engineering design process, highlighting that engineers use this cycle to solve problems.
- Present today’s challenge: “You are going to take on the role of a chemical engineer. We need to make cleaner drinking water for all people in our community. Today, you are going to create a water filtration system that removes impurities from dirty water collected from a local water source.”
- Split students into groups.
- Show students the materials available them: pebbles (large and small), sand (fine and coarse), coffee filters, cotton balls, a plastic bottle with the bottom cut off, a plastic cup, and carbonated charcoal (optional for all groups).
- Give each team time to brainstorm and plan how they might separate components of the water using the available materials. Have students draw their plan in their Filtration System Design Packet and answer the questions in the Design Your Filtration System section.
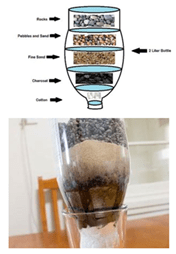
- Once each team has a plan, have them present it to you for approval.
- After gaining approval, each group should build their filtration system, starting with their cut-off bottle and carefully layering the materials according to their plan.
- After building their filtration system, each group member should answer the questions in the Build Your System section of their Filtration System Design Packet.
- Let students pour their dirty water through their filtration systems, collecting the filtered water in the plastic cup below.
- Have students label and cover their filtered water samples.

Day 4: Test and Improve: Filtration system evaluation
- Explain that chemical engineers test and evaluate their prototype designs to make improvements. “Yesterday, you designed a filtration system to purify water from a local body of water. Today, we will evaluate and redesign our systems to ensure we have the cleanest water.”
- Have students complete the Analyze Results section of their Filtration System Design Packet.
- Instruct students to work together to plan how they might improve their filtration system. Their plan should include what filtering materials are available to use. Encourage students to think about how each material will help filter the water.
- Have students write down their plan in the Redesigning and Retesting Your Filtration System section of their Filtration System Design Packet.
- Have each group present their improvement plan to you for approval.
- After gaining approval, let students rebuild their filtration system.

- Let students pour their dirty water through their filtration system, collecting the filtered water in the plastic cup below.
- Give students time to answer the questions in the Redesigning and Retesting Your Filtration System section of their Filtration System Design Packet.
- After filtering the water, have students discuss their systems with another group. Ask them to compare their systems and determine which system worked better. Encourage them to think about what made the better system more effective.
- Give students time to answer the questions in the Reflection section of their Filtration System Design Packet; optionally, assign as homework.


Day 5: Iteration: Filtering colored water through activated charcoal
- Explain that students will further develop their filtration system to remove PFAS. To simulate PFAS, food coloring will be added to water samples.
- Show the video “Engineers Develop Water Filtration System That Permanently Removes ‘Forever Chemicals’” from NBC News (4:14 minutes): https://www.youtube.com/embed/ov7FTzDQy-k.
- Hand out one Removal of PFAS Worksheet to each student.
- Instruct students to brainstorm and plan how to separate the colored water. Their plan must include the use of activated charcoal, two cotton balls, a plastic water bottle with the base removed, water from the previous experiment, and two small plastic cups.
- Have students draw their group plan in the Plan section of the Removal of PFAS Worksheet
- Once students have a plan, have them present it to you for approval.
- Have each team build their PFAS filtration device using the provided materials.
- Once each team has built their PFAS filtration device, announce the challenge: Each team must remove the PFAS (represented by food coloring) from their water samples. Teams can run the water through their device as many times as necessary, using the tools provided. All teams will start at the same time.
- Set a timer for 5 minutes.
- Let teams work to filter their water sample through their filter for 5 minutes.
- At the end of the 5-minute timer, have one member from each team bring their filtered water sample to the front of the room.
- Select one student to be the official tester.
- Have the official tester test each sample with the water quality tester and declare the winner.
- Once a winner is declared, move one member from each team to an area for a discussion. Give these new groups time to discuss their designs and outcomes.
- Have students return to their original groups and discuss ways to improve their filtration system design based on the feedback and test results.
- If time allows, allow teams to rebuild their filtration devices and retest them using the same process.
- Have students complete the Removal of PFAS Worksheet with their teams and turn it in when finished.
Vocabulary/Definitions
chemical engineering: The design and development of chemical manufacturing processes. Chemical engineers apply the principles of chemistry, physics, and engineering to design equipment and processes for manufacturing products such as gasoline, detergents, and paper.
environmental engineering: A professional discipline concerned with protecting people from adverse environmental effects, as well as protecting ecosystems and improving the quality of the environment.
filtration: A process to remove particles from a liquid.
perfluoroalkyl and polyfluoroalkyl substances (PFAS): A large, complex group of synthetic chemicals that have been used in consumer products around the world since the 1950s. They are ingredients in various everyday products.
separation: The process of separating or extracting different components of a mixture using some physical methods.
water pollution : Contamination of a body of water.
Assessment
Pre-Activity Assessment
Notice and Wonder Sheet: Students look at the map of PFAS contamination in the United States and write down what they notice and wonder. They then share their answers with their shoulder partners and in a classroom discussion.
Activity Embedded (Formative) Assessment
Filtration System Design Packet: Students follow the engineering design process to imagine, plan, and create a water purification system. They then test their prototypes and analyze their results.
Post-Activity (Summative) Assessment
Rubric: Student designs are evaluated by the Water Filtration Rubric.
Troubleshooting Tips
- Prepare cups that have carbonated charcoal, water samples, sand, and pebbles premeasured.
- Put small holes in the cups that will be used to collect the water after it is filtered through the charcoal.
- Assemble two plastic bottles, with one cut in half and the other taped together.
- Label the plastic cups with the location from which the students will collect water on Day 1.
Activity Extensions
Students can research how the water filtration system in their community works. How does it compare to other community systems? Students can identify sources of PFAS and the impact on communities with large numbers.
Compare different water from a variety of water bottle companies using PPM reader https://www.wecofilters.com/tds-ez.html.
Allow one group to use carbonated charcoal during the Day 1 design.
Put a price tag on each material used. Look at making the best design with the lowest price.
Activity Scaling
Scaling can be done through teacher-led demonstrations. Students can observe a teacher-led demonstration of the water filtration system and the filtration through the carbonated charcoal to remove the colored water.
Subscribe
Get the inside scoop on all things TeachEngineering such as new site features, curriculum updates, video releases, and more by signing up for our newsletter!More Curriculum Like This

Students are presented with examples of the types of problems that environmental engineers solve, specifically focusing on water quality issues. Topics include the importance of clean water, the scarcity of fresh water, tap water contamination sources, and ways environmental engineers treat contamin...
Copyright
© 2025 by Regents of the University of Colorado; original © 2024 University of KansasContributors
Ryan Stidham; Abbie Peters; Charles Ponge; Dr. Mark Shifflett; Dr. Douglas Huffman; Dr. Meagan Patterson; Dr. Prajna DharSupporting Program
IDEA-BioE RET Program through the University of KansasAcknowledgements
This material is based upon work supported by the National Science Foundation under grant no. ECC-2055716 - a Research Experience for Teachers program titled Inquiry-Driven Engineering Activities using Bioengineering (IDEA-BioE) at the University of Kansas. Any opinions, findings and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation.
Last modified: December 15, 2025








User Comments & Tips