Quick Look
Grade Level: 9 (9-12)
Time Required: 2 hours 45 minutes
(Divided into three 55-minute sessions:
Day 1 – Setup
Day 2 – Data collection
Day 3 – Post-lab analysis and discussion)
Expendable Cost/Group: US $0.50
Group Size: 2
Activity Dependency: None
Subject Areas: Earth and Space
NGSS Performance Expectations:

| HS-ESS1-3 |
Summary
In this activity, students model how scientists must rely on and collaborate with engineers in order to make new scientific discoveries, such as a new star-planet system if “Earth were no longer an option.” Building off the ideas students previously established about what makes Earth and the sun ideal for sustaining life, students begin the engineering design process by brainstorming ideas for how they can determine what something is made of and what tools that would require. Students are then challenged to think whether their method would still work if that “something” is too far away (or deadly) to bring into a lab! After re-evaluating the problem, students are introduced to the basic principles of spectroscopy. By building their own spectrometers using a computer, Arduino, and a SparkFun Spectroscopy Sensor, students are able work through the fundamentals of spectroscopy in a hands-on experience. This activity has students use spectrometry data that they obtained to differentiate among common lab substances, and then identify an unknown substance. They gain practice using technology, interpreting graphs, creating emission spectra diagrams, and forming scientific arguments. Afterward, students apply what they have learned to determine the element composition of stars located in our galaxy and predict which stars have potential for sustaining life.Engineering Connection
Scientists ask questions, experiment, and make discoveries. But to do so, they need specific tools. Thus, “Engineering Tools for Scientific Discovery” is one of the 14 Grand Challenges presented by the National Academy of Engineering. In this activity, students act as scientists and as engineers, demonstrating how each depends on the other. As engineers, students think about how to determine the composition of an item, brainstorm what tools could be used, evaluate whether those tools work for an item that is far away (i.e., a star), and build a spectrometer. Then, as scientists, they use the spectrometer to observe absorption spectra for various materials, and then identify an unknown substance.
Learning Objectives
After this activity, students should be able to:
- Justify why collaboration between scientists and engineers is essential in designing specific tools for scientific discovery.
- Utilize a simple spectrometer to gather emission spectra data.
- Describe that atoms of each element emit and absorb unique patterns of frequencies (wavelengths) of light that allow us to identify them.
Note: This activity does not focus on HOW atoms absorb and emit light energy, only that they do.
- Interpret and analyze emission/absorption spectra data to determine the elemental composition of an item.
Note: This includes data captured in the lab setting and provided data such as star spectral diagrams.
Educational Standards
Each TeachEngineering lesson or activity is correlated to one or more K-12 science,
technology, engineering or math (STEM) educational standards.
All 100,000+ K-12 STEM standards covered in TeachEngineering are collected, maintained and packaged by the Achievement Standards Network (ASN),
a project of D2L (www.achievementstandards.org).
In the ASN, standards are hierarchically structured: first by source; e.g., by state; within source by type; e.g., science or mathematics;
within type by subtype, then by grade, etc.
Each TeachEngineering lesson or activity is correlated to one or more K-12 science, technology, engineering or math (STEM) educational standards.
All 100,000+ K-12 STEM standards covered in TeachEngineering are collected, maintained and packaged by the Achievement Standards Network (ASN), a project of D2L (www.achievementstandards.org).
In the ASN, standards are hierarchically structured: first by source; e.g., by state; within source by type; e.g., science or mathematics; within type by subtype, then by grade, etc.
NGSS: Next Generation Science Standards - Science
-
DCI.ESS1.A.9-12.6.
The study of stars' light spectra and brightness is used to identify compositional elements of stars, their movements, and their distances from Earth.
(Grades 9 - 12)
More Details
Do you agree with this alignment?
-
DCI.PS4.B.9-12.10.
Atoms of each element emit and absorb characteristic frequencies of light. These characteristics allow identification of the presence of an element, even in microscopic quantities.
(Grades 9 - 12)
More Details
Do you agree with this alignment?
| NGSS Performance Expectation | ||
|---|---|---|
|
HS-ESS1-3. Communicate scientific ideas about the way stars, over their life cycle, produce elements. (Grades 9 - 12) Do you agree with this alignment? |
||
| Click to view other curriculum aligned to this Performance Expectation | ||
| This activity focuses on the following Three Dimensional Learning aspects of NGSS: | ||
| Science & Engineering Practices | Disciplinary Core Ideas | Crosscutting Concepts |
| Communicate scientific ideas (e.g. about phenomena and/or the process of development and the design and performance of a proposed process or system) in multiple formats (including orally, graphically, textually, and mathematically). Alignment agreement: | The study of stars' light spectra and brightness is used to identify compositional elements of stars, their movements, and their distances from Earth. Alignment agreement: Other than the hydrogen and helium formed at the time of the Big Bang, nuclear fusion within stars produces all atomic nuclei lighter than and including iron, and the process releases electromagnetic energy. Heavier elements are produced when certain massive stars achieve a supernova stage and explode.Alignment agreement: | In nuclear processes, atoms are not conserved, but the total number of protons plus neutrons is conserved. Alignment agreement: |
Materials List
Teacher-specific resources:
- masking tape or file folder stickers/labels, to label samples
- sharpie, to label samples
- What’s In Our Stars? Introduction Presentation
- What’s In Our Stars? Teacher Procedures Presentation
- What’s In Our Stars? Sample Tracker
- What’s In Our Stars? Lab Handout Answer Key
Each student needs:
- What’s In Our Stars? Lab Handout
- colored pencils (can be shared), one for each color in ROYGBIV plus gray
- black pen or thin black marker
Each group needs:
- laptop computer
- What’s In Our Stars? Excel Dashboard
- What’s In Our Stars? Arduino Code
- spectrometer:
- 1 Spectroscopy Sensor, available online here
- 1 Qwiic to Breadboard Connection Cable, available online here
- 1 Arduino Uno Rev3, available online here
- 1 Arduino to USB 2.0 cable, available online here
- 1 piece of black construction or heavy cardstock cut to approximately 8 cm x 8 cm (~3” x 3”)
- 1 pair of scissors
- 1 set of samples:
- 3 different substances of known identity in sample cups/cuvettes
- 1 substance of unknown identity in sample cup/cuvette
For the entire class to share:
- teacher’s choice of 3 different substances to use as “test samples,” enough to give each group a complete set of samples. A complete set includes 1 of sample A, 1 of sample B, 1 of sample C, and 1 “unknown” that is the same substance found in either sample A, B, or C.
- See Before the Activity for further explanation, example group set, and suggested substances.
- To hold samples:
- What’s In Our Stars? Student Procedures Presentation
Worksheets and Attachments
Visit [www.teachengineering.org/activities/view/mis-2824-stars-spectrometer-spectroscopy-activity] to print or download.Pre-Req Knowledge
Students should have a basic understanding of elements, the electromagnetic spectrum, and wavelengths. Students also will need to be able to read and interpret a data table, a bar graph, and a line graph.
Introduction/Motivation
(Show What’s In Our Stars? Introduction Presentation Slides 8-12. Ensure that students have a copy of the What’s In Our Stars? Lab Handout to follow along with the text below.)
As we hunt for Earth 2.0, a key aspect that needs to be considered is what star our new planet is going to be orbiting. There are trillions (or more!) of stars just in our Milky Way Galaxy. So, how do we as scientists begin to determine what makes a star ideal for sustaining life? The first step is to discover how stars differ from one another. As you look into the night sky, you can see that they differ in size and brightness. But stars also differ in elemental composition (i.e., what elements are in them). The way scientists determine what elements are in materials that are too far (or…deadly) to bring into an actual lab is through spectroscopy.
Spectroscopy studies the pattern of wavelengths of light that an atom absorbs or emits when energized. Each element has a unique “fingerprint” of wavelengths that they will absorb/emit. This information is incredibly valuable, because different materials interact with light in different ways. By analyzing the patterns of wavelengths that interact with an object, scientists can identify the material, study its properties, and even detect substances that are otherwise invisible to the naked eye.
However, to determine those wavelengths, we need specific tools—this is where engineers come in! Scientists can’t detect those patterns of wavelengths just by looking at an object with the naked eye; they need a tool that can recognize each wavelength and determine the intensity (i.e., the amount of energy) of each wavelength. Engineers make this possible by designing and building devices called spectrometers. There are many different types of spectrometers, varying in price, sensitivity, and method of detection, but the end goal is the same: to empower scientists to make new discoveries and form a deeper understanding of the world (and universe) around us.
In this lab, you will take on the role of an engineer and build a spectrometer! Then, you will take on the role of a scientist and use the spectrometer to determine a unique spectral diagram for various lab materials, and then use those diagrams to identify an unknown substance. Last, you’ll apply the same concept and process to determine the composition of various stars in our galaxy!
Procedure
Background
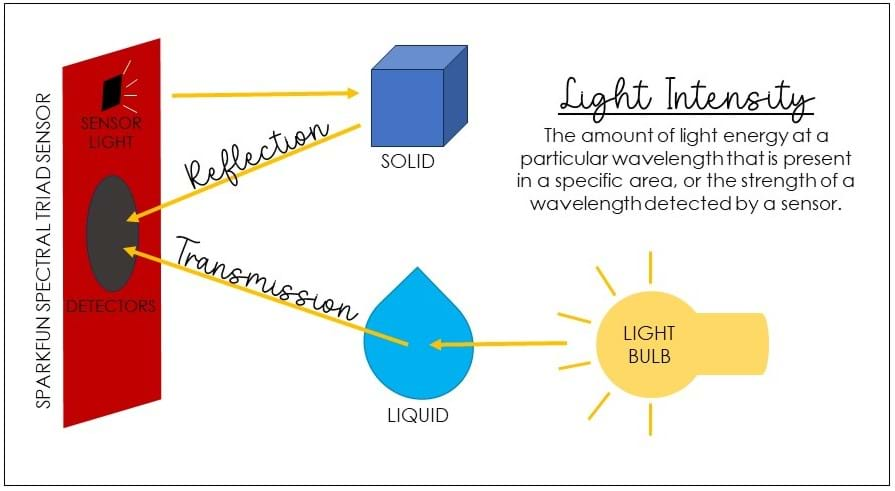
The SparkFun Triad Spectroscopy Sensor (AS7265x) does not directly measure the amount of light energy reflected off an object. Instead, it measures the intensity of light within specific wavelength ranges in the visible spectrum. This can indirectly provide information about the light reflected off an object if the object interacts with or absorbs light within those wavelength ranges.
When light interacts with an object, it can be absorbed, transmitted through, or reflected by the object's surface as shown in Figure 1. 
For example, certain materials may absorb light at specific wavelengths, resulting in lower intensity readings in those channels. Other materials may reflect light strongly at certain wavelengths, leading to higher intensity readings in those channels. By comparing the spectral patterns to known references or doing data analysis, researchers and/or engineers can gain insights into the composition of the object or material under investigation.
The sensor can be used for a variety of applications, including color sensing, material identification, and environmental monitoring, leveraging the unique spectral signatures of different substances. However, it's important to note that this sensor measures the intensity of light within predefined wavelength ranges rather than directly quantifying the amount of light energy reflected off an object. Also, when investigating liquid samples, it is the amount of light energy from a secondary light source that is transmitted through the liquid. Therefore, as concentration increases, the average intensity will decrease, as more of the light energy is being absorbed versus transmitted. Additionally, there is likely to be some error due to the scattering of light as it passes through the container.
Before the Activity
- Gather all materials.
- Cut the black construction or heavy cardstock cut to squares approximately 8 cm x 8 cm (~3” x 3”).
- Familiarize yourself with the computer prep steps (see Procedure Part 1 and What’s In Our Stars? Teacher Procedures Presentation Slides 5-10).
- Add links and Arduino code to Google Classroom (or similar location for students to access):
- The link to download and install the Arduino software
- The code What’s In Our Stars? Arduino Code
- The link to download and install the Microsoft Data Streamer Add-in
- Ensure that students have access to Microsoft Excel on their computers.
- Prepare samples for each group. (See What’s In Our Stars? Teacher Procedures Presentation Slides 16-19 for more information).
- Optional: Use the What’s In Our Stars? Sample Tracker to help keep track of the substances and samples prepared for each group.
- Select three materials for the samples; each group needs three known and one unknown sample (the “unknown” sample should be the same as one of the known samples so students can identify it). For solid samples, it is recommended that the samples look similar to the naked eye (e.g., table salt, Epsom salt, and sugar). Ideally, sample substances would be made from elements hydrogen (Z= 1) to iron (Z=26), as these elements are formed due to fusion within stars.
- Example substances: table salt, baking soda, table sugar, cornstarch, vegetable oil, vinegar, etc. (See What’s In Our Stars? Teacher Procedures Presentation Slide 17 for more ideas.)
- Place solid samples in 2 oz plastic containers and liquid samples in 3.5 mL cuvettes. Fill the container approximately halfway with the sample. For each group, label the known sample containers with the name of the sample materials, and label the last container “unknown.”
- To provide more guidance to students during the activity, prepare to show the What’s In Our Stars? Student Procedures Presentation or provide access to the presentation via Google Classroom.
With the Students
DAY 1:
Part 1:
- Introduce the activity using the Introduction and Motivation section. Have students complete the post-background questions on the second page of the What’s In Our Stars? Lab Handout.
- Lead students through the process of setting up the computer programs/files for the activity. Show What’s In Our Stars? Student Procedures Presentation Slides 3-9 to provide visuals of the computer prep process.
- Have students follow the Part 1 instructions on the third page of the What’s In Our Stars? Lab Handout.
- Provide guidance to students as needed. A summary of the process:
- Have students download and install the Arduino software using the link in Google Classroom.
- Once installed, have students open Arduino IDE and add the SparkFun Library.
- Have students download the code What’s In Our Stars? Arduino Code from Google Classroom and open in Arduino IDE.
- Instruct students to open the What’s In Our Stars? Data Dashboard in Microsoft Excel found in Google Classroom.
- Have students download and install the Microsoft Data Streamer add-in using the link found in Google classroom.
Part 2:
- Separate students into groups of two to three students.
- Show What’s In Our Stars? Student Procedures Presentation Slides 10-17 to provide visuals for Part 2.
- Have students follow the Part 2 directions on the third page of the What’s In Our Stars? Lab Handout.
- Provide guidance to students as needed. A summary of the process:
- Have students connect the Qwiic cable to the spectrometer sensor.
- Have students connect the jumper cables to the Arduino Uno, making sure they are in the correct spot; see the diagram on Slides 12-13 of the What’s In Our Stars? Student Procedures Presentation.
- Have students connect the Arduino Uno to the computer/device using the USB cable.
- Allow students to grab the samples for testing; each group should have three known and one unknown sample cups.
- Have students remove the plastic top (as appropriate) and place the square paper cover onto the top of their first known sample cup.
- Have students place the spectrometer face down in the center of the cover over the first sample cup.

Students prepare to collect data for their samples using the SparkFun Spectral Triad (AS7265x) Sensor and Arduino Uno R3 as a spectrometer.
DAY 2:
Part 3:
- Show What’s In Our Stars? Student Procedures Presentation Slides 18-30 to provide visuals for Part 3.
- Have students follow the Part 3 directions on the third page of the What’s In Our Stars? Lab Handout. Provide guidance to students as needed; see the Troubleshooting Tips and Slides 26-28 of the What’s In Our Stars? Teacher Procedures Presentation or Slides 22-24 of the What’s In Our Stars? Student Procedures Presentation for fixes to possible errors to the data dashboard. A summary of the process:
- Have students upload the code (opened in Arduino IDE) to the Arduino Uno.
- Have students go to the Data Streamer tool bar in Excel and connect the device.
- Have students check that the settings in the Excel data dashboard are correct; if not, instruct students to see the troubleshooting notes on Slides 22-24 of the What’s In Our Stars? Student Procedures Presentation.
- Have students select the name of their first known sample from the dropdown menu on the dashboard.
- Have students begin data streaming.
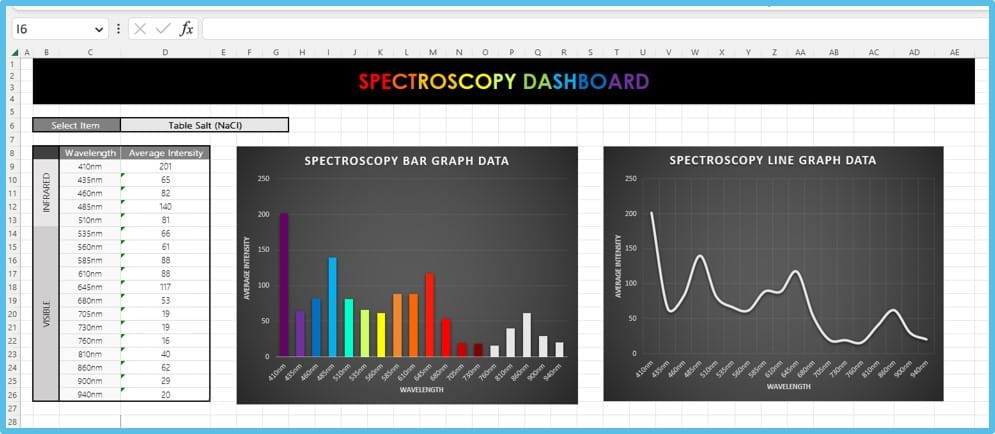
An example of an Excel dashboard once data has been collected. - Have students stop data streaming after approximately 10 seconds.
- Have students save the file in the documents folder using one of the methods shown in the slides.
- Have students record the data by drawing a black line using a marker or pen on the spectra diagrams on the handout at the wavelengths where a “peak” appears on the line graph.
- Have students reset the dashboard and their Arduino Uno.
- Have students repeat steps for the two remaining known samples and one unknown sample.
- Ensure that students are carefully recording their data on the What’s In Our Stars? Lab Handout and that each group member has a chance to collect and record data.
Part 4a:
- Have students follow Steps 1-3 in the Part 4 directions on the third page of the What’s In Our Stars? Lab Handout.
- Check that students carefully return their samples to the front of the room (or another central location). A summary of the process:
- Have students disconnect and disassemble the spectrometer.
- Have students return their samples, making sure the tops are securely on each of the cups.
- Have students close Arduino IDE and the Excel dashboard.
DAY 3:
Part 4b:
- Show What’s In Our Stars? Student Procedures Presentation Slides 31-33 to provide visuals for Part 4b.
- Have students complete the remaining portions of the What’s In Our Stars? Lab Handout.
Vocabulary/Definitions
absorption spectrum: The unique pattern of specific wavelengths of light that are absorbed by a substance when interacting with light.
emission spectrum: The unique pattern of specific wavelengths of light that are emitted (given off) by a substance when heated or energized.
light intensity: The amount of light energy at a particular wavelength that is present in a specific area, or the strength of a wavelength detected by a sensor.
spectrometer: A scientific instrument used to measure and analyze the properties of light (such as intensity and wavelength) when interacting with a substance.
spectroscopy: A scientific technique that allows scientists to identify a substance by examining and analyzing the unique properties of the substance when interacting with light.
Assessment
Pre-Activity Assessment
Bell-Ringer/Warm-up Question:
At the beginning of class, give students time to students answer the question “How do you know what something is made of?” Encourage students to pick any object and think about what they would DO to figure out what the object is made of. Remind them this is an independent brainstorming session and there aren’t any wrong answers! Once a few minutes have passed, instruct students to share their ideas with a partner or small group, and then discuss ideas as a class.
After discussing the methods students come up with, depending on time, have students further discuss how they would determine the composition of something that they can’t see (such as air) or that is too far away (such as the sun). Ask what types of equipment or tools they would need to find an answer. (It doesn’t have to be a specific tool or equipment that students already know; an example answer could be as simple as “something that can sort types of atoms.”) After having a class discussion, introduce the concept of spectroscopy and spectrometers as a method scientists use to answer this very question!
For convenience, a page within the student lab handout is provided for students to write their own ideas and ideas of others for the questions mentioned above.
Provided Materials:
Activity Embedded (Formative) Assessment
“What’s In Our Stars?” Lab – Known Samples
During the “What’s In Our Stars?” lab activity, students set up and use a spectrometer to gather absorption spectra data at specific wavelengths of visible and infrared light. Using the data collected, they create absorption spectral diagrams of three known substances and one unknown substance. As students work, ensure that they complete the lab handout and work with their groups.
Provided Materials:
Post-Activity (Summative) Assessment
“What’s In Our Stars?” Lab – Unknown Sample
Once they’ve completed the data collection and processing for the three known samples, students examine a sample of an “unknown” substance. Using the same procedure for the unknown sample as the known samples, students collect data and create a spectral diagram for the unknown sample. Students then compare their spectral diagrams to identify the unknown sample. Monitor students as they work; ensure that they can explain their conclusions for what the unknown substance is.
Provided Materials:
“What’s In Our Stars?” Lab – Analysis Questions
Students complete analysis questions located at the end of the student handout. These questions ask students to use the skills practiced in the lab to interpret and analyze absorption spectral diagrams of various elements and stars. Students are then asked to determine what elements are found within the stars given. Use the lab handout answer key to review student responses.
Provided Materials:
Safety Issues
Safety concerns will depend on the sample substances the teacher chooses to use.
- Review proper safety protocols on the safety data sheets for the chosen substances.
- Follow proper disposal procedures for the chosen substances.
- Use eye protection (goggles or safety glasses) and gloves if using any acids or bases (liquid or anhydrous).
- Have students wash their hands after completing the lab to ensure any chemical residue is removed.
Troubleshooting Tips
During the data collection process, students may encounter errors with the data table. See Slides 26-28 in the What’s In Our Stars? Teacher Procedures Presentation for troubleshooting tips.
Additional Multimedia Support
- Computer Applications Used:
- Arduino IDE (Cloud or Desktop)
- Microsoft Excel
- Microsoft Excel Data Streamer Add-in
- Microsoft PowerPoint
- LMS (Google Classroom, Canvas, Schoology, etc.)
- Optional Videos:
- Spectroscopy of Stars - Wonders of the Universe: Stardust - BBC Two (4:16 minutes)
- 200 years of Fraunhofer lines (4:03 minutes)
Subscribe
Get the inside scoop on all things TeachEngineering such as new site features, curriculum updates, video releases, and more by signing up for our newsletter!More Curriculum Like This

Students are introduced to the basic known facts about the universe, and how engineers help us explore the many mysteries of space.
References
Kramida, A., Ralchenko, Yu., Reader, J. and NIST ASD Team (2022). NIST Atomic Spectra Database (version 5.10), [Online]. Available: https://physics.nist.gov/asd. National Institute of Standards and Technology, Gaithersburg, MD. DOI: https://doi.org/10.18434/T4W30F
Kristelle Bougot-Robin, Jack Paget, Stephen C. Atkins, and Joshua B. Edel. “Optimization and Design of an Absorbance Spectrometer Controlled Using a Raspberry Pi To Improve Analytical Skills”. Journal of Chemical Education. (2016) Vol.93, No.7, pp. 1232-1240. DOI: 10.1021/acs.jchemed.5b01006
National Academy of Engineering. 2023. Engineer the tools of Scientific Discovery Grand Challenges - Engineer the Tools of Scientific Discovery. Available at: http://www.engineeringchallenges.org/challenges/discovery.aspx
OpenAI. ChatGPT. OpenAI, 2021https:/www.openai.com.
Seidle, Nathan (Nate). Spectral Triad (AS7265x) Hookup Guide, [Online]. Available: https://learn.sparkfun.com/tutorials/spectral-triad-as7265x-hookup-guide?_ga=2.208195537.274963032.1659022095-1771007873.1658366162. SparkFun Electronics. Licensed by CC BY-SA 4.0
“Spectroscopy 101 – Introduction”. James Webb Space Telescope. 7 July 2021, NASA, ESA. webbtelescope.org/contents/articles/spectroscopy-101--introduction.
Copyright
© 2024 by Regents of the University of Colorado; original © 2023 Michigan State UniversityContributors
Christina Abbott, Matt Jourdan, Leyf StarlingSupporting Program
Research Experience for Teachers (RET), Michigan State University College of EngineeringAcknowledgements
This curriculum was developed through the Michigan State University College of Engineering NSF RET program under grant number CNS-1854985 under National Science Foundation. However, these contents do not necessarily represent the policies of the NSF, and you should not assume endorsement by the federal government.
Special thanks to Dr. Zhen Qui and Aniwat Juhong, at Michigan State University Institute for Quantitative Health Sciences and Engineering Lab 4400. Thank you to the Research Experience for Teachers Principal Investigators -Dr. Wen Li and Drew Kim for offering this program in conjunction with Michigan State University College of Engineering. And thank you to Matt Jourdan and Leyf Starling for your support, guidance, and assistance in developing this activity.
Last modified: October 19, 2024






User Comments & Tips