Quick Look
Grade Level: 10 (9-12)
Time Required: 3 hours
(can be split into 2-3 sessions)
Expendable Cost/Group: US $5.00 The activity also uses non-expendable items such as Arduino UNO microcontrollers, breadboards, LCD displays and computers; see the Materials List for details.
Group Size: 3
Activity Dependency: None
Subject Areas: Chemistry, Science and Technology
NGSS Performance Expectations:

| HS-ETS1-2 |
| HS-PS1-3 |
Summary
Student groups construct simple conductivity probes and then integrate them into two different circuits to test the probe behavior in solutions of varying conductivity (salt water, sugar water, distilled water, tap water). The activity culminates with student-designed experiments that utilize the constructed probes. The focus is to introduce students to the fabrication of the probe and expose them to two different ways to integrate the probe to obtain qualitative and quantitative measurements, while considering the application and utility of a conductivity probe within an engineering context. A provided handout guides teams through the process: background reading and questions; probe fabrication including soldering; probe testing and data gathering (including circuit creation on breadboard); probe connection to Arduino (including circuit creation and code entry) and a second round of testing and data gathering; design and conduct their own lab experiments that use the probes; online electrolyte/nonelectrolyte reading, short video, comprehension check and analysis questions.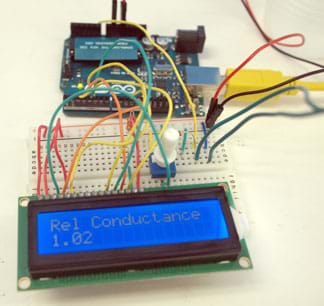
Engineering Connection
Engineers often design sensors to fit their exact needs. Engineers planning solar panel installations require sensors that measure the amount of light falling on a given area. If engineers are dealing with aqueous or liquid solutions, it is quite common to characterize a solution's conductivity to gain insight into the types of solutes dissolved within the solution (either ionic or covalent solutes). Chemical engineers often create systems to transport fluids, so an understanding of fluid properties is important to the design process. In designing a desalination plant, engineers must be able to measure the amount of salt present in seawater and in the fresh water produced; a conductivity probe is a valuable instrument to identify the presence or absence of ionic compounds.
Through this lab, students are exposed to multiple ways that a conductivity probe can be used and challenged to consider its possible real-world applications. The primary function of the probe is to indicate the ease with which electrical current can flow through a solution; the easier current flows, the higher the conductivity. This single measurement determines whether an aqueous solution contains ionic or covalent solutes, an important first step in characterizing unknown solutions.
Learning Objectives
After this activity, students should be able to:
- Identify whether a solution is conductive.
- Construct a functional conductivity probe.
- Integrate a conductivity probe into a simple circuit.
- Utilize an Arduino with LCD display-to-display probe values.
- Determine the conductivity of an unknown solution.
Educational Standards
Each TeachEngineering lesson or activity is correlated to one or more K-12 science,
technology, engineering or math (STEM) educational standards.
All 100,000+ K-12 STEM standards covered in TeachEngineering are collected, maintained and packaged by the Achievement Standards Network (ASN),
a project of D2L (www.achievementstandards.org).
In the ASN, standards are hierarchically structured: first by source; e.g., by state; within source by type; e.g., science or mathematics;
within type by subtype, then by grade, etc.
Each TeachEngineering lesson or activity is correlated to one or more K-12 science, technology, engineering or math (STEM) educational standards.
All 100,000+ K-12 STEM standards covered in TeachEngineering are collected, maintained and packaged by the Achievement Standards Network (ASN), a project of D2L (www.achievementstandards.org).
In the ASN, standards are hierarchically structured: first by source; e.g., by state; within source by type; e.g., science or mathematics; within type by subtype, then by grade, etc.
NGSS: Next Generation Science Standards - Science
| NGSS Performance Expectation | ||
|---|---|---|
|
HS-ETS1-2. Design a solution to a complex real-world problem by breaking it down into smaller, more manageable problems that can be solved through engineering. (Grades 9 - 12) Do you agree with this alignment? |
||
| Click to view other curriculum aligned to this Performance Expectation | ||
| This activity focuses on the following Three Dimensional Learning aspects of NGSS: | ||
| Science & Engineering Practices | Disciplinary Core Ideas | Crosscutting Concepts |
| Design a solution to a complex real-world problem, based on scientific knowledge, student-generated sources of evidence, prioritized criteria, and tradeoff considerations. Alignment agreement: | Criteria may need to be broken down into simpler ones that can be approached systematically, and decisions about the priority of certain criteria over others (trade-offs) may be needed. Alignment agreement: | |
| NGSS Performance Expectation | ||
|---|---|---|
|
HS-PS1-3. Plan and conduct an investigation to gather evidence to compare the structure of substances at the bulk scale to infer the strength of electrical forces between particles. (Grades 9 - 12) Do you agree with this alignment? |
||
| Click to view other curriculum aligned to this Performance Expectation | ||
| This activity focuses on the following Three Dimensional Learning aspects of NGSS: | ||
| Science & Engineering Practices | Disciplinary Core Ideas | Crosscutting Concepts |
| Plan and conduct an investigation individually and collaboratively to produce data to serve as the basis for evidence, and in the design: decide on types, how much, and accuracy of data needed to produce reliable measurements and consider limitations on the precision of the data (e.g., number of trials, cost, risk, time), and refine the design accordingly. Alignment agreement: | The structure and interactions of matter at the bulk scale are determined by electrical forces within and between atoms. Alignment agreement: | Different patterns may be observed at each of the scales at which a system is studied and can provide evidence for causality in explanations of phenomena. Alignment agreement: |
International Technology and Engineering Educators Association - Technology
-
Use appropriate symbols, numbers, and words to communicate key ideas about technological products and systems.
(Grades
3 -
5)
More Details
Do you agree with this alignment?
-
Apply the technology and engineering design process.
(Grades
6 -
8)
More Details
Do you agree with this alignment?
State Standards
New York - Science
-
Define a simple design problem reflecting a need or a want that includes specified criteria for success and constraints on materials, time, or cost.
(Grades
3 -
5)
More Details
Do you agree with this alignment?
-
Plan and conduct an investigation to gather evidence to compare the structure of substances at the bulk scale to infer the strength of electrical forces between particles.
(Grades
9 -
12)
More Details
Do you agree with this alignment?
Materials List
Each group needs:
- laptop or desktop computer with USB cable and Internet access
- 2 x 20 cm lengths of 22 gauge single-strand insulated copper wire; available at hardware stores
- 2 x 10 cm lengths of 32 gauge nichrome wire; such as from Amazon
- wire stripper
- plastic barrel from a disposable pen, such as a BIC pen
- electrical tape
- four plastic cups, for the four test solutions
- half-size or larger breadboard, such as the reasonably priced boards at Amazon
- assorted jumper wires, such as a pack of 30 7-inch wires (PRT-11026) at Sparkfun
- 470 Ω resistor, such as the resistor multipacks at Amazon
- red LED, such as the basic red 5 mm LED (COM-09590) at Sparkfun
- 9V battery
- 2 x 9V battery connector, such as the heavy-duty 9V snap connectors at Amazon
- Arduino UNO, such as the Rev3 or equivalent
- 16 x 2 LCD display, such as the basic 16-character by 2-line display with black text on green background (LCD-00255 ROHS) at Sparkfun, or equivalent
- 10K Ω trimpot (aka trimming potentiometer, a small variable resistor), such as the trimpot 10K with knob (COM-09806) at Sparkfun
- 10K Ω resistor, such as the resistor multipacks at Amazon
- 220 Ω resistor, such as the resistor multipacks at Amazon
- Build and Test a Conductivity Probe Lab Handout, one per person
To share with the entire class:
- soldering iron(s), such as at Amazon
- lead-free solder, a few tubes to share among one class, such as at Amazon
- table salt, 250 g
- sugar, 250 g
- spoons or spatulas
- wax pencil or tape and marker, to label the cups
- distilled water, expect 1 gallon to be enough for an entire class
- tap water and sink drain
- paper towels, for drying the rinsed probes
- additional supplies may be needed to enable students to use their conductivity probes to conduct end-of-activity experiments of their own designs, for example, solutions of common household chemicals, lemon juice, sodium hydroxide, ammonia, at varying concentrations and temperatures
Worksheets and Attachments
Visit [www.teachengineering.org/activities/view/nyu_probe_activity1] to print or download.Pre-Req Knowledge
Students should have the following skills before beginning this lab activity:
- A basic understanding of soldering*
- How to use a breadboard to construct simple circuits
- Be able to identify the LED, resistor, jumper wire and related electrical components used in the activity
*For students with no soldering experience, refer to the SparkFun "How to Solder" tutorial (written description with photographs and a three-minute video) at: https://learn.sparkfun.com/tutorials/how-to-solder---through-hole-soldering?_ga=1.165393596.20730219.1441226013
In addition, students should have an understanding of the following chemistry concepts:
- Ionic and covalent compounds
- Aqueous solutions, including how to make a simple solution
- Basic lab safety for conducting experiments in a chemistry laboratory
Introduction/Motivation
Have you ever wondered why you have to get out of the swimming pool when a thunderstorm approaches? It has everything to do with conductivity—the ability of a solution to conduct electrical current. A solution conducts electrical current when charged particles (ions) are in the aqueous solution. The movement of these positive and negative ions enables the flow of charge through the solution.
In this lab activity, we will make a probe to use as a tool to determine very quickly whether a solution conducts electricity (ZAP!!) or not. Fundamentally, the probe measures the resistance to the flow of charge and must be submerged within the testing solution to work. If the solution contains ions, charge will be able to flow easily through the probe (the resistance will be low). However, if the solution contains only water molecules or a solution of water and other covalent solutes, no charged particles exist to facilitate the flow of charge so the resistance across the probe will be very high.
Once we have built the probes, we will look at two different ways to display the results—using light as well as an LCD numerical display. Afterwards, you will build on your understanding of how the conductivity probe works to design and conduct an experiment of your own.
Procedure
Background
This lab activity requires students to make simple solutions by dissolving various solutes into measured volumes of water. Depending upon the solute, the resulting solution will either conduct electricity or be non-conductive. Conductive solutions always result from the presence of a solute that is ionic, meaning it dissociates in water into charged particles called ions. When an ionic substance dissociates, both positive cations and negative anions form, and both are necessary to enable a solution conduct an electrical current. The conductivity of a solution depends upon the amount of ions present; therefore, more concentrated ionic solutions have a higher conductivity than more dilute solutions. Outside of the chemistry classroom, students may have heard of "electrolytes" and "nonelectrolytes," words that are synonyms for ionic compounds and covalent compounds, respectively.
The measurement of conductivity is typically a measure of how easily electrical current is able to move through a solution. The higher the conductivity of a solution, the less resistance to the flow of electrical current. This is the basis for the conductivity probe that students fabricate and use. The probe measures resistance and translates the resistance as an indicator of conductivity. Hence, if the solution has a high resistance, such as with a nonelectrolytic solute, the conductivity will be very low and if the solution has a low resistance, such as with an electrolytic solute like table salt, the conductivity will be very high. The conductivity probe uses simple electrical circuits to communicate the measurement to the user.
- In the first experiment, students receive only qualitative feedback from the probe in the form of a LED lighting up (if the solution is conductive) or remaining unlit (if the solution is non-conductive).
- In the second experiment, the feedback comes in the form of a digital numerical readout (constituting a quantitative measurement) in which, as the numerical value displayed increases, the conductivity of the solution increases.
From an engineering point-of-view, conductivity probes are frequently utilized in situations in which water quality monitoring is essential. For example, desalination facilities use conductivity measurements to ensure that water samples have been sufficiently purified. Conductivity probes are also used to quickly identify the source of contaminants within wastewater streams and water supply systems. Another common application involves the use of conductivity probes to monitor the ionic content of water used within cooling towers since too highly concentrated ionic solutions can prematurely corrode components and cause safety hazards.
Refer to the following Emerson Process Management application data sheet, Theory and Application of Conductivity, for an overview of typical ways conductivity is utilized in industrial processes.
Timing note: Plan on 40 minutes for Parts 1 and 2, building and testing the probe; 80 minutes for Part 3, connecting the probe to the Arduino; and 60 minutes for Part 4, critical thinking and analysis; for a total of 180 minutes.
Before the Activity
- Gather materials and make copies of the Build and Test a Conductivity Probe Lab Handout (or distribute to students as a digital file).
- Set-up student lab stations with the supplies necessary for each group.
- Make sure students will have easy computer Internet access to the online reading and digital experiment for the critical thinking and analyzing portion of the activity (see links on the handout and in the Additional Multimedia Support section), especially the CK-12 links that require (free) account access.
With the Students—Introduction/Team Formation
- Present the Introduction/Motivation content to the class.
- Review what is necessary in order to form a solution, that is, a solute dissolved into a solvent.
- Pass out the handouts and direct students to individually answer the four "preparing" questions on page 2. Once students have finished answering the questions, review their responses as a class before starting the lab activity, as described in the Assessment section. (optional/alternative approach: Have students share their responses prior to sorting into groups to start the experiment. Do NOT correct student predictions at this point.)
- Divide the class into groups of three or four students each.
With the Students—Part 1: Constructing the Probe
- Direct groups to collect the materials to complete Part 1, which are listed on page 3 of the handout. The supplies for Part 1 are shown in Figure 1.

Figure 1. Supplies for Part 1, to fabricate the probe. - Have groups proceed to construct their conductivity probes. The basic steps entail twisting the nichrome wire onto the exposed end of one insulated copper wire and then soldering the nichrome wire to the insulated wire, and repeating. Then the two wires are taped onto opposite sides of the pen barrel. Refer to Figures 2 and 3.

Figure 2. An example stripped wire. 
Figure 3. An example wrapped wire.
- Using wire strippers, remove about 1 cm of insulating material from the ends of each of the two lengths of insulated copper wire.
- Next, twist one of the nichrome wires onto the exposed copper end of one of the insulated wires. Then solder the wires together to make a strong connection, as seen in Figure 4.

Figure 4. Two wires soldered together. - Tape the soldered wires onto the pen barrel, leaving a 1 mm section of the nichrome wires exposed near the tip, so that the probe can make physical contact with the solution in which it will be immersed (see Figures 5 and 6).

Figure 5. Placing the wires before taping. 
Figure 6. Wires taped to a plastic pen barrel. - Use electrical tape to cover the entire probe, with the exception of the 1 mm gap near the tip; see Figures 7 and 8).

Figure 7. Using tape to cover the entire probe except for a 1 mm gap. 
Figure 8. The finished probe.
With the Students—Part 2: Testing the Probe
- Following the handout instructions, groups each create a simple circuit using a breadboard, LED, 470 Ω resistor, 9V battery with wires and some jumper wires. Refer to Figure 9 for the completed circuit and Figure 10 for the circuit schematic.

Figure 9. Conductivity sensor testing setup. 
Figure 10. A schematic of the LED circuit.
- As students complete their breadboard circuits, require them to have the teacher check their circuits. Since the LED will not illuminate unless it is immersed in a solution that contains an electrolyte, have a cup of salt water handy to quickly test the circuits. Look for common circuitry mistakes related to the LED. The LED must have its long leg connected to the positive battery end.
- Have groups proceed to follow the handout instructions to each make two solutions—salt water and sugar water. Both are simple to make; students stir in a spoonful of either salt or sugar into a cup filled halfway with distilled water. Using distilled water—not tap water—is crucial for these solutions, particularly for the sugar water solution.
- Once the circuits are checked by the teacher, students test their probes by immersing them into cups of four different solutions: salt water, sugar water, distilled water and tap water. Remind students to rinse the probe with distilled water between trials because it is extremely easy to contaminate solutions with even a small amount of liquid. Advise students to rinse the probe with distilled water and dry between tests.
- Once groups have completed recording their results in Data Table 1 of the handout for Part 2, make sure they save their solutions for use later in Part 3.
With the Students—Part 3: Connecting the Probe to the Arduino
- As students begin Part 3, each group uses a computer connected to an Arduino UNO to upload its codes to the Arduino. Have students follow the handout instructions to gather supplies and proceed.
- One of the most challenging components of this activity is the correct construction and wiring of the LCD screen to the Arduino. Advise students to take their time building the breadboard circuitry and frequently reference Figure 11, which is Figure 3 on the handout. Figure 12 is the corresponding circuit schematic. Note: Although Figure 11 does not show the trimpot connected to the GND and the anode, it should be connected as shown in Figure 12.

Figure 11. Circuitry for connecting the probe to the Arduino UNO. Note: Although Figure 11 does not show the trimpot connected to the GND and the anode, it should be connected as shown in Figure 12. 
Figure 12. Circuit schematic corresponding to the circuitry shown in Figure 11. - It is important that the trimpot used in this circuit is used solely to adjust the contrast on the LCD screen. When groups show you their circuit boards to check the connections, make sure that the trimpot is in the middle of its range of motion. This ensures that the text and numerical values are visible on the LCD display when the code runs for the first time after students upload it.
- Once groups have constructed their circuitry and had it verified by the teacher, they open the Arduino software on their computers and use a USB cord to connect the Arduino to the computer. They enter the code provided on the handout (or copy/paste the code if a digital version of the handout is made available). Alternatively, you may provide students with the Conductivity Sensor with LCD ino file that contains the code so that they can merely open the file, verify the contents and upload to the Arduino. The code is shown in Figure 13, which is Figure 4 on the handout. Since it is often helpful to explain the meaning of the code to students, refer to Arduino Code Comments for the Teacher, which provides a screen capture of the code with comments that explain what each line of the code does.

Figure 13. Conductivity measurement code for the Arduino. - Once students have successfully run the code, expect them to see the LCD power on and display the text: Rel Conductance. Point out that this value is related directly to how easily electrical current can pass through the solution. The more easily that current flows, the higher the conductivity value displayed on the screen.
- Then students follow the handout instructions to repeat the testing of the four solutions they created in Part 2, this time recording in Data Table 2 of the handout the numerical relative conductivity values displayed on the LCD screen.
- Once students have finished testing the solutions, direct them to dispose of all solutions in a sink drain and clean up their lab work spaces.
With the Students—Part 4: Critical Thinking and Analysis
- During this final component of the lab activity, students work together to complete the "critical thinking" portion of the lab activity as presented on the handout. This requires groups to design additional experiments that utilize their conductivity sensors. Encourage students to feel free to design experiments that use the Part 2 or Part 3 lab activity setup. Examples of what students might test:
- Monitor conductivity while an acid is added to a base, such as lemon juice added to a dilute solution of sodium hydroxide or ammonia.
- Measure the conductivity of various common household chemicals.
- Measure the conductivity of solutions of varying concentrations.
- Measure the conductivity of a solution at various temperatures.
- For all proposed group experiments, make sure that the procedures include safety considerations and use reasonable (readily available and not dangerous) solutions. Proofread (and modify as necessary) all proposed procedures before permitting groups to proceed to conduct their designed lab experiments.
- Next, students complete the "analyzing" section of the lab activity, composed of an online reading about electrolytes and nonelectrolytes, including a six-minute video, followed by an online digital experiment to test their reading comprehension. Then they answer three handout questions.
- Conclude by facilitating a class discussion to share, compare and distill experiences, results and conclusions. See a few example discussion questions in the Assessment section.
Vocabulary/Definitions
conductivity: The degree to which a specified material conducts electricity, calculated as the ratio of the current density in the material to the electric field that causes the flow of current. It is the reciprocal of the resistivity.
current: A quantity representing the rate of flow of electric charge, usually measured in amperes.
electrolyte: A substance that ionizes when dissolved in suitable ionizing solvents such as water.
nonelectrolyte: A substance that does not readily ionize when dissolved or melted and is a poor conductor of electricity.
probe: A small device, especially an electrode, used for measuring, testing or obtaining information.
qualitative: Relating to, measuring or measured by the quality of something rather than its quantity.
quantitative: Relating to, measuring or measured by the quantity of something rather than its quality.
solute: The minor component in a solution, dissolved in the solvent.
solution: A liquid mixture in which the minor component (the solute) is uniformly distributed within the major component (the solvent).
solvent: The liquid in which a solute is dissolved to form a solution.
Assessment
Pre-Activity Assessment
Preparing: Before beginning the lab activity, direct students to individually answer the four questions in the "preparing" section of the Build and Test a Conductivity Probe Lab Handout, on page 2. Then review and discuss students' responses to the questions to make sure they understand the role of the probe, its real-world applications and the types of substances that the probe helps to identify as being present or absent within solutions being analyzed. Encourage students to make additions/modifications to their handout answers as a result of the discussion. If time permits, ask students to think of other ways that conductivity probes might be useful; for example, engineers often use conductivity probes for clean-in-place processes, detection of pipe leaks and chemical titrimetric analysis.
Activity Embedded Assessment
Check for Understanding: As students progress through Parts 1, 2 and 3, expect them to be able to describe how they know that a solution is conductive.
- Part 1: As students construct their probes, take time to discuss how the probe works in order to make sure they realize that the test solution is part of the circuit since it is the connecting medium between the two nichrome wires. This portion of the lab is primarily fabrication, so ensure that all students understand the probe construction process.
- Part 2: In this section, it is important for students to think about the type of solution that they have created (electrolyte or nonelectrolyte) and see if their results make sense with what they know about the solution being tested. As a prompt to test for understanding, ask: Did your results match what you expected? Alternatively, have all student groups present their Part 2 results and discuss whether their results make sense. Take this opportunity to reinforce types of solutes (ionic and covalent), as well as resulting solutions being electrolytic or nonelectrolytic.
- Part 3: Parts 2 and 3 are much the same in terms of testing, but how the probe's measurement is communicated is very different. While working, prompt students to explain the key difference in learning that a solution is conductive in Part 3, compared to Part 2. Expect them to observe that the numerical readout in Part 3 permits finer conductivity measurement. To foreshadow the upcoming critical thinking challenge (in which groups design and conduct their own experiments using the probes), ask students how they might use the Part 3 setup to test other solutions by asking: What else could you test, and why?
Post-Activity Assessment
Analyzing: Direct students to complete the "analyzing" section of the lab activity, composed of an online reading about electrolytes and nonelectrolytes, including a six-minute video, followed by an online digital experiment to test their reading comprehension. Then they answer three handout questions. Review their answers to gauge their depth of comprehension.
Concluding Class Discussion: At activity end, lead a class discussion to share, compare and distill experiences, results and conclusions. Example discussion questions:
- Why did tap water have a measureable conductivity in Part 3, but not light the LED in Part 2? (Answer: The Part 3 setup provides a much more sensitive measurement of conductivity than that of Part 2. Though both measurements are for the same solution, the means of communicating conductivity [light in Part 2 and a numerical measurement display in Part 3] are very different.)
- What other uses might your Part 3 sensor setup serve? (Answer: In addition to identifying solutions as electrolytic or nonelectrolytic, the sensor could differentiate between solutions of differing concentrations.)
Safety Issues
When soldering the wires in Part 1, require students to wear safety glasses and be sure they know that a soldering iron is extremely hot, even for several minutes after it is unplugged.
Troubleshooting Tips
When working on Part 2, remind students that the LED is a one-way device and will not work if they reverse the installation of the LED on the breadboard. Make sure the longer leg of the LED is connected to the positive voltage source.
Activity Scaling
When using these materials with younger (grades 9-10) students who have limited coding experience, provide them with the Arduino file, Conductivity Sensor with LCD code, since entering the code can be a frustration point.
Additional Multimedia Support
During the activity, students require access to the following Internet resources:
- Theory and Application of Conductivity article https://www.emerson.com/documents/automation/application-data-sheet-theory-application-of-conductivity-rosemount-en-68442.pdf
- Electrolytes and Nonelectrolytes article, including a six-minute video: http://www.ck12.org/chemistry/Electrolytes-and-Nonelecrolytes/lesson/Electrolytes-and-Nonelectrolytes-CHEM/
- A digital experiment to check comprehension of the previous article: http://www.ck12.org/assessment/tools/geometry-tool/plix.html?eId=SCI.CHE.432.4&questionId=53ceca67da2cfe48ba6cfc9a&artifactID=1817915&backUrl=http%3A//www.ck12.org/chemistry/Electrolytes-and-Nonelecrolytes/%23interactive
In addition, the CK-12 Foundation has an excellent little web app that serves as an appropriate follow up for students after completing the activity. It also includes a simple embedded model of a conductivity sensor setup and five questions for students on the topic of electrolyte solutions and conductivity.
Subscribe
Get the inside scoop on all things TeachEngineering such as new site features, curriculum updates, video releases, and more by signing up for our newsletter!More Curriculum Like This

Students gain an understanding of the difference between electrical conductors and insulators, and experience recognizing a conductor by its material properties. In a hands-on activity, students build a conductivity tester to determine whether different objects are conductors or insulators.

Students are introduced to the technology of flexible circuits, some applications and the photolithography fabrication process. They are challenged to determine if the fabrication process results in a change in the circuit dimensions since, as circuits get smaller and smaller (nano-circuits), this c...

Students learn about nondestructive testing, the use of the finite element method (systems of equations) and real-world impacts, and then conduct mini-activities to apply Maxwell’s equations, generate currents, create magnetic fields and solve a system of equations. They see the value of NDE and FEM...

Students learn about current electricity and necessary conditions for the existence of an electric current. Students construct a simple electric circuit and a galvanic cell to help them understand voltage, current and resistance.
Copyright
© 2015 by Regents of the University of Colorado; original © 2015 Polytechnic Institute of New York UniversityContributors
Phillip Cook, Iulian IriminaSupporting Program
SMARTER RET Program, Polytechnic Institute of New York UniversityAcknowledgements
This activity was developed by the Science and Mechatronics Aided Research for Teachers with an Entrepreneurial ExpeRience (SMARTER): A Research Experience for Teachers (RET) Program in the School of Engineering funded by National Science Foundation RET grant no. 1132482. However, these contents do not necessarily represent the policies of the NSF, and you should not assume endorsement by the federal government.
Last modified: July 17, 2023









User Comments & Tips