Quick Look
Grade Level: 5 (3-5)
Time Required: 45 minutes
Expendable Cost/Group: US $0.50
Group Size: 2
Activity Dependency: None
Subject Areas: Earth and Space
Summary
Students learn about ocean currents and the difference between salt and fresh water. Using colored ice cubes, they see how cold and warm water mix and how this mixing causes currents. Students also learn how surface currents occur due to wind streams, how fresh water floats on top of salt water, the difference between water in the ocean and fresh water throughout the planet, and how engineers are involved in the design of ocean water systems for human use.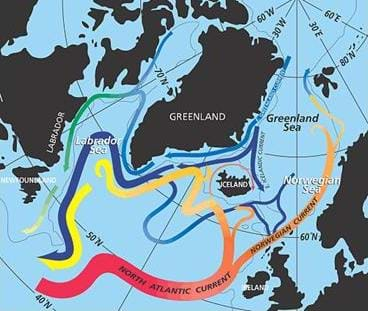
Engineering Connection
As resources on land become more scarce, engineers are looking to the ocean to meet human needs for food, minerals and transportation. Engineers develop systems for operation on and in the ocean. A huge area of interest for ocean engineering is hydrodynamics and acoustics—to communicate underwater using sound. To design with sound, engineers must understand the fluid dynamics of the ocean and sound waves. Engineers also work on many other projects involving the ocean, including the design of seaports, ships and submarines. They work with geologists to design better equipment for drilling oil deep on the ocean floor, and with marine biologists to monitor natural ecosystems within the ocean. Other engineers design equipment that can measure the temperature and depth of the ocean from space.
Learning Objectives
After this activity, students should be able to:
- Compare and contrast the properties of salt water in the oceans/seas and freshwater elsewhere on the planet.
- Describe mixing caused by currents in the ocean, including the effects of warm and cold water as well as surface currents.
- List several systems that engineers are designing that involve the ocean as a resource.
Educational Standards
Each TeachEngineering lesson or activity is correlated to one or more K-12 science,
technology, engineering or math (STEM) educational standards.
All 100,000+ K-12 STEM standards covered in TeachEngineering are collected, maintained and packaged by the Achievement Standards Network (ASN),
a project of D2L (www.achievementstandards.org).
In the ASN, standards are hierarchically structured: first by source; e.g., by state; within source by type; e.g., science or mathematics;
within type by subtype, then by grade, etc.
Each TeachEngineering lesson or activity is correlated to one or more K-12 science, technology, engineering or math (STEM) educational standards.
All 100,000+ K-12 STEM standards covered in TeachEngineering are collected, maintained and packaged by the Achievement Standards Network (ASN), a project of D2L (www.achievementstandards.org).
In the ASN, standards are hierarchically structured: first by source; e.g., by state; within source by type; e.g., science or mathematics; within type by subtype, then by grade, etc.
NGSS: Next Generation Science Standards - Science
-
Nearly all of Earth's available water is in the ocean. Most fresh water is in glaciers or underground; only a tiny fraction is in streams, lakes, wetlands, and the atmosphere.
(Grade 5)
More Details
Do you agree with this alignment?
International Technology and Engineering Educators Association - Technology
-
Explain how various relationships can exist between technology and engineering and other content areas.
(Grades
3 -
5)
More Details
Do you agree with this alignment?
State Standards
Colorado - Science
-
Develop and communicate an evidence-based scientific explanation for changes in weather conditions
(Grade
5)
More Details
Do you agree with this alignment?
Materials List
Each group needs:
- Oceans Away Worksheet, one per person
Each group needs for Activity 1 – Follow that Current
- 2 colored ice cubes (colored with dark food coloring; prepared in advance by the teacher)
- clear baking dish or see-through container
- warm or hot water
Each group needs for Activity 2 – Currents in the Wind
- 1 spice, such as oregano, that shows up in clear water
- clear baking dish or see-through container (may be the same container used in activity 1)
- room-temperature water
Each group needs for Activity 3 – Fresh Water Floats?
- salt
- food coloring (dark colors work best)
- 2 clear glass jars or other container that enables students to see a clear lineation between the salt and fresh water (should be taller than the baking dish in activities 1 and 2)
- room-temperature water
To share with the entire class:
- paper towels, for cleanup
- spoons, for adding salt to water and stirring in food coloring
- pitcher(s), to pour water into baking dishes and cups
- ice tray, to make ice cubes
- access to a freezer
Worksheets and Attachments
Visit [www.teachengineering.org/activities/view/cub_earth_lesson2_activity2] to print or download.Introduction/Motivation
Have you ever looked at a map of the world or a globe? (If possible, show a globe or world map as a prop.) Look at all the water on the Earth. A lot of the bodies of water are connected to each other. In fact, the world's oceans are really one large world ocean. Where on Earth do you think the water is warmer or colder? (Answer: Warmer near the equator; colder ner the poles.) What do you think happens when the cold water from the north and south poles meets the warm water from the equator? (Listen to student ideas.) Well, the answer is currents. Currents are movements of water in oceans (or lakes). The ocean's water currents move continuously around the Earth, which keeps its temperatures stable. How does the ocean help keep the temperatures of the Earth regular? Well, the ocean water affects the temperature of the atmosphere in part by absorbing incoming radiation from the sun, and then the ocean currents mix the warm and cool waters together, keeping the temperatures from getting too hot.
Did you know that the oceans make up about 97% of the water on Earth? That's a lot of water! How many oceans are on our planet? Can you name them? (Listen to student ideas.) You have probably heard of these four oceans: Pacific, Atlantic, Indian and Arctic. In 2000, the International Hydrographic Organization announced a new ocean, the Southern Ocean, which surrounds Antarctica and extends up to 60 degrees latitude.
Have you ever tasted ocean water? Well, it's very salty! It is not something you would want to drink due to its high salt content. The amount of salt in water is called its salinity. The salinity of the oceans and seas are different throughout the world, but the average salt content is 2.2 pounds of salt for every cubic foot of water. The saltiest seawater is in the Red Sea and the Persian Gulf region, which is 40 o/oo (parts per thousand) salt. The least salty water is in the polar regions, where the water is mixed with melting ice and heavy precipitation. Consider an iceberg that sits in the ocean for centuries. It is composed of fresh or salt water? (Answer: Icebergs are formed when parts of the Antarctic ice sheet break off. The ice sheet, which covers the continent, is formed by snow and ice falling on inland areas, thus icebergs are freshwater.)

What do you know about the ocean? Today, we are going to look at some properties of fresh water (in our case, water from the tap) and ocean water. We are going to learn about how water mixes and how salt water moves in fresh water. These are important concepts for engineers to understand when designing any kind of system that deals with ocean water.
Procedure
Before the Activity
- Gather materials and make copies of the Oceans Away Worksheet.
- Make dark-colored ice cubes for Activity 1 – Follow that Current.
With the Students
Activity 1 – Follow that Current
- Fill up baking dishes three-quarters of the way to the top with warm/hot tap water.
- Give each group 2 colored ice cubes.
- Ask students to observe what happens to the colored ice cubes. Where does the colored water go? Have them write descriptions of what they see on their worksheets.
Activity 2 – Currents in the Wind
- Fill up the baking dishes three-quarters of the way to the top with room-temperature tap water. (Or, if continuing from Activity 1, use the resulting water.)
- Sprinkle a small amount of spice over the water.
- Direct students to lightly blow over the surface of the water.
- Ask students what happens to the spices as you blow over the surface of the water? Have them write their answers on their worksheets.
Activity 3 – Fresh Water Floats?
- Hand out 2 glasses to each student group.
- Fill the first glass three-quarters full with room-temperature tap water.
- Fill the second glass one-quarter full with room-temperature tap water.
- Have students add 1 spoonful of salt to the second glass (the one with less water) and stir. Direct them to continue to add salt until no more salt dissolves in the water (expect 1 or 2 spoonfuls to be enough).
- Have students add 1 or 2 drops of food coloring to the salt water.
- Pour the colored salt water slowly into the glass containing the clear fresh water. Observe what happens.
- What happened to the salt water on top? Did it sink or float? Have students write their answers on their worksheets.
- If time permits, do the activity again except put colored salt water in first and add clear fresh water on top. What happens to the fresh water on top? Does it sink or float?
Vocabulary/Definitions
current : A tidal or non-tidal movement of lake or ocean water.
equator: The imaginary line on the Earth's surface that exactly divides the Earth into northern and southern hemispheres.
freshwater: When referring to a resource, water that is specifically not salty.
poles: Refers to the North and South Poles, the areas at the furthermost north and south points on the planet Earth.
salinity : A measure of the amount of salt something contains.
Assessment
Pre-Activity Assessment
Discussion Questions: Solicit, integrate and summarize student responses. Ask the students:
- Would you expect water on different parts of the planet to be warmer in some places than others? Why?
- Where do you think ocean water would be warm/cold?
- How is ocean water different than water you find in lakes and streams?
Activity Embedded Assessment
Group Question: During the activity, ask the groups:
- Why do you think the colored water from the ice cube moves to the bottom of the pan of warm water? Do you think a similar movement might also happen in the ocean? (Answer: As the warm water rises, the cold water sinks and this movement causes underwater currents.)
- How does wind cause surface currents? (Answer: Wind blows over the surface and causes currents that move around the surface water.)
- Does salt water float on top of fresh water, or does salt-water sink into the fresh water? (Answer: Salt water sinks into the fresh water because salt water is heavier than fresh water. Therefore, fresh water floats on top of salt water.)
Post-Activity Assessment
Question/Answer: Ask the students and discuss as a class:
- What is a current? (Answer: The movement of water in an ocean or lake.)
- What are two causes of currents? (Answer: Temperature differences and wind.)
- Does salt water float on top of fresh water, or sink? (Answer: It sinks. This happens because salt water is denser than fresh water.)
- Would you want to drink water from the ocean? Why or why not? (Answer: No, it is very salty and would eventually dehydrate you due to the consumption of too much salt.)
Engineering Ocean Water: Assign students generate a list of all the different things they can think of that use water. (Examples may include daily activities such as brushing teeth, drinking, watering plants, cleaning dishes, as well as other things like ships, submarines, dams, fish tanks and swimming pools.) Next, have them circle all of the items on their lists that use salty ocean water. Then, discuss with the students the remaining items on their lists. Expect that most items on their lists require fresh water. Engineers are designing systems that take the salt out of ocean water and turn it into fresh water. Have students circle items on their lists that might be able to use ocean water if the salt were removed. (Expect them to circle all items now.) Their second set of circles show why it is important for engineers to understand the properties of the ocean as a resource, especially with the small amount of fresh water that is available for use and the high demand of activities that need fresh water every day.
Safety Issues
Do not run the risk of burning students by using use water that is too hot; warm to hot water from the tap is sufficient.
Troubleshooting Tips
Have supplies ready and pitchers of water filled to make the transitions between activities run smoothly.
When students are directed to blow lightly across their baking dishes of water, remind them not to spit or blow hard. Blowing too hard easily causes the water leave the dish and blow onto other students.
Activity Extensions
Have students mark up a map of the world's oceans with the major ocean currents, labeling them as a surface or underwater currents.
Activity Scaling
- For upper grades, set up the activity as an example of how to use the scientific process. Have students write hypotheses, define their variables, conduct the experiment and then state their conclusions.
- For lower grades, conduct the three activities as demonstrations or stations so students do not have to do as much setup.
Subscribe
Get the inside scoop on all things TeachEngineering such as new site features, curriculum updates, video releases, and more by signing up for our newsletter!More Curriculum Like This

Students learn about water bodies on our plant, including their uses and qualities. They learn several ways that engineers help people to maintain and conserve water sources.

Students explore characteristics that define climatic regions. They learn how tropical, desert, coastal and alpine climates result in different lifestyles, clothing, water sources and food options for the people who live there.
References
Jenner, Lynn. National Aeronautics and Space Administration, Life on Earth, "Looking at Earth," February, 28, 2006. Accessed September 18, 2020. http://www.nasa.gov/images/content/143186main_giss_map_lg.gif
Copyright
© 2006 by Regents of the University of ColoradoContributors
Sara Born; Malinda Schaefer Zarske; Janet YowellSupporting Program
Integrated Teaching and Learning Program, College of Engineering, University of Colorado BoulderLast modified: September 28, 2020







User Comments & Tips