Quick Look
Grade Level: 10 (8-11)
Time Required: 45 minutes
Expendable Cost/Group: US $5.00 The activity also requires the non-expendable cost to make one conductivity meter per group (or shared between groups or the class, if necessary) using a BASIC Stamp microcontroller, a timer and other supplies, estimated at $53 each. Alternatively, use standalone conductivity meters or modified multimeters.
Group Size: 3
Activity Dependency: None
Subject Areas: Science and Technology
NGSS Performance Expectations:

| HS-PS1-3 |

Summary
Students use conductivity meters to measure various salt and water solutions, as indicated by the number of LEDs (light emitting diodes) that illuminate on the meter. Students create calibration curves using known amounts of table salt dissolved in water and their corresponding conductivity readings. Using their calibration curves, students estimate the total equivalent amount of salt contained in Gatorade (or other sports drinks and/or unknown salt solutions). This activity reinforces electrical engineering concepts, such as the relationship between electrical potential, current and resistance, as well as the typical circuitry components that represent these phenomena. The concept of conductors is extended to ions that are dissolved in solution to illustrate why electrolytic solutions support the passage of currents.Engineering Connection
Electrical engineers employ the fundamental knowledge of electricity in their work, and our industrial society greatly benefits from the work that they do! The efficient transport of electrons through electrolyte solutions is critical for applications such as fuel cells, water treatment facilities, electrochemical cells and batteries, as well as for the biological functioning of living organisms. In biological systems, electrolytes (among other solutes) are dissolved with various body fluids to enable many processes within organisms. However, over time, the amount of these dissolved solutes become depleted and must be replenished through the ingestion of food. Under times of extreme exertion, such as playing sports for long periods of time, individuals may suffer from dehydration, which is the excessive loss of body fluids (such as water) and electrolytes. As a precaution, athletes commonly ingest sports drinks such as Gatorade to prevent the excessive loss of bodily fluids to prevent the onset of dehydration. Since sports drinks contain different types of electrolytes, this activity is designed to measure the total amount of electrolytes contained in Gatorade, by equating the measurements of Gatorade to solutions of known amounts of sodium chloride.
Learning Objectives
After this activity, students should be able to:
- Use conductivity to estimate the amount of salt in Gatorade.
- Explain how electricity travels through liquids.
Educational Standards
Each TeachEngineering lesson or activity is correlated to one or more K-12 science,
technology, engineering or math (STEM) educational standards.
All 100,000+ K-12 STEM standards covered in TeachEngineering are collected, maintained and packaged by the Achievement Standards Network (ASN),
a project of D2L (www.achievementstandards.org).
In the ASN, standards are hierarchically structured: first by source; e.g., by state; within source by type; e.g., science or mathematics;
within type by subtype, then by grade, etc.
Each TeachEngineering lesson or activity is correlated to one or more K-12 science, technology, engineering or math (STEM) educational standards.
All 100,000+ K-12 STEM standards covered in TeachEngineering are collected, maintained and packaged by the Achievement Standards Network (ASN), a project of D2L (www.achievementstandards.org).
In the ASN, standards are hierarchically structured: first by source; e.g., by state; within source by type; e.g., science or mathematics; within type by subtype, then by grade, etc.
NGSS: Next Generation Science Standards - Science
| NGSS Performance Expectation | ||
|---|---|---|
|
HS-PS1-3. Plan and conduct an investigation to gather evidence to compare the structure of substances at the bulk scale to infer the strength of electrical forces between particles. (Grades 9 - 12) Do you agree with this alignment? |
||
| Click to view other curriculum aligned to this Performance Expectation | ||
| This activity focuses on the following Three Dimensional Learning aspects of NGSS: | ||
| Science & Engineering Practices | Disciplinary Core Ideas | Crosscutting Concepts |
| Plan and conduct an investigation individually and collaboratively to produce data to serve as the basis for evidence, and in the design: decide on types, how much, and accuracy of data needed to produce reliable measurements and consider limitations on the precision of the data (e.g., number of trials, cost, risk, time), and refine the design accordingly. Alignment agreement: | The structure and interactions of matter at the bulk scale are determined by electrical forces within and between atoms. Alignment agreement: | Different patterns may be observed at each of the scales at which a system is studied and can provide evidence for causality in explanations of phenomena. Alignment agreement: |
Common Core State Standards - Math
-
Solve linear equations and inequalities in one variable, including equations with coefficients represented by letters.
(Grades
9 -
12)
More Details
Do you agree with this alignment?
-
Represent data on two quantitative variables on a scatter plot, and describe how the variables are related.
(Grades
9 -
12)
More Details
Do you agree with this alignment?
-
Use units as a way to understand problems and to guide the solution of multi-step problems; choose and interpret units consistently in formulas; choose and interpret the scale and the origin in graphs and data displays.
(Grades
9 -
12)
More Details
Do you agree with this alignment?
-
For a function that models a relationship between two quantities, interpret key features of graphs and tables in terms of the quantities, and sketch graphs showing key features given a verbal description of the relationship.
(Grades
9 -
12)
More Details
Do you agree with this alignment?
International Technology and Engineering Educators Association - Technology
-
Explain how knowledge gained from other content areas affects the development of technological products and systems.
(Grades
6 -
8)
More Details
Do you agree with this alignment?
State Standards
New York - Science
-
Plan and conduct an investigation to gather evidence to compare the structure of substances at the bulk scale to infer the strength of electrical forces between particles.
(Grades
9 -
12)
More Details
Do you agree with this alignment?
Materials List

Each group needs:
- ~5 grams sodium chloride (if not available, use Kosher salt), to make its own calibration solutions
- ~2.5 - 3 liters water, to make its own set of calibration solutions
- 7-8 cups or beakers (this includes individual cups for the solutions needed for the calibration curve, plus 1-2 cups for the unknown solutions)
- BASIC Stamp conductivity meter (created by teacher, in advance, see below); alternatively, use a standalone conductivity meter or a multimeter (set to measure resistance, with a few modifications using a spacer and some tape, see the Procedure section)
- ruler
- Conductivity of Gatorade Worksheet, one per student
To share with the entire class:
- ~500 ml Gatorade of each of three (or more) flavors, enough of each to adequately immerse the probe
- ~500 ml pickle juice, any kind, enough to adequately immerse the probe
- sodium chloride (or Kosher salt), to make ~500 ml of an "unknown" salt solution for students to test

The BASIC Stamp conductivity meter, a device created by the teacher in advance of the activity.
To make a BASIC Stamp conductivity meter (estimated cost: $53):
- BASIC Stamp Activity Kit - Serial + USB (Text v.3.0) ($50, includes the HomeWork Board with a built-in BASIC Stamp 2 microcontroller, LEDs, wires, resistors and capacitors; item code 90005, available from parallax.com)
- computer (system requirements for PC: Pentium 133 mhz or higher, 32 MB RAM, VGA display; for MAC: G3 or higher with 64 MB RAM, color display)
- BASIC Stamp editor software (latest version for your operating system, free download available from Parallax Inc at http://www.parallax.com/Support/DownloadsPress/tabid/477/Default.aspx)
- (5) 550 Ω resistors (included in BASIC Stamp Activity Kit)
- 20 kΩ resistor (included in BASIC Stamp Activity Kit)
- 1 μF capacitor (included in BASIC Stamp Activity Kit)
- 22 AWG wire (included in BASIC Stamp Activity Kit)
- (5) LEDs, any color (included in BASIC Stamp Activity Kit)
- 555 Timer IC CMOS ($2 each, item code: 604-00009; available from parallax.com)
- (2) 5-inch 4/40 stainless steel screws
- (4) 4/40 nylon nuts
- roll of electrical tape
- hack saw
- Fisherbrand® SureOne™ pipette tip rack (100μl–1000μl size) (This is the empty container that is usually thrown out once all the tips are used. For this activity, it serves as a neat and tidy housing for the circuitry. As such, it is difficult to put a price on it; a case of these tips costs $65 and includes 10 racks.)
- BD Falcon 50 ml conical centrifuge tube with screw caps ($337 per case of 500, catalog number: 14-432-24; available from Fisher Scientific [www.fishersci.com])
- (4) zip ties
- drill, to make two holes in the cap
- Conductivity Meter Building and Calibration Instructions
Worksheets and Attachments
Visit [www.teachengineering.org/activities/view/nyu_gatorade_activity1] to print or download.Pre-Req Knowledge
Students should have a basic understanding of circuits and electricity.
Introduction/Motivation
The properties of electricity and the work of electrical engineers appear in so many places in your everyday lives. In our ever-changing world, a continual drive exists to make our conventional methods of producing and storing electricity more economical and environmentally friendly. Devices designed by engineers, such as fuel cells and electric motors, show promise for future applications such as in automobiles. As such, developing media that can store, support and effectively propagate the flow of electrons is (and will continue to be) a vital area of research in our society.
Today, you will get to explore some of the fundamental concepts that electrical engineers use in this work by measuring the conductivity of Gatorade!
Procedure
Background
The BASIC Stamp conductivity meters needed for this activity are built by the teacher before the activity using the provided instructions (they are highly reusable). Using these meters, conductivity is indicated by the number of LEDs (light emitting diodes) that light up. The BASIC Stamp conductivity meter is constructed using a BASIC Stamp microcontroller and a 555 timer (in a stable multi-vibrator mode, in which the conductivity meter is modeled as one of the resistors). As the resistance of the solution changes with the addition of salt, the frequency of the 555 timer's output changes accordingly. The conductivity meter is programmed such that the output of the 555 timer controls the activation of the LEDs, in which more LEDs are turned on with solutions of higher salt concentrations.
This activity can be conducted using any conductivity meter, so instead of the BASIC Stamp conductivity meter, you can use either of the following:
- a standalone electrical conductivity meter, which is the traditional type of conductivity meter (~$20-150); it typically displays the value of conductivity in seimens per meter
- a multimeter (~$10-300), a device that can measure electrical resistance; so, in this case, electrical resistivity is recorded instead of number of LEDs or conductivity; if using multimeters, make the modifications described in the Procedure section
The activity is presented assuming one conductivity meter per group, but depending on the availability of materials and class size, the meter(s) could be shared among the groups or the class. This is left to the discretion of the teacher.
Using the conductivity meter, students record the conductivity of various solutions of water and known quantities of salt. Once completed, they use the resulting calibration curves to estimate the equivalent amounts of salt that are contained in an unknown solutions, such as Gatorade or other sports drinks.
Before the Activity
- Gather supplies and make copies of the Conductivity of Gatorade Worksheet.
- Build enough BASIC Stamp conductivity meters (or acquire standalone conductivity meters or multimeters) so you have one per student group (unless deciding to share amongst groups or the class). Refer to the Conductivity Meter Building and Calibration Instructions for details.
- If using multimeters (set to measure resistance), make the following modifications:
- Keep the distance between the multimeter probes (leads) constant throughout the experiment by using a spacer and some tape.
- The concentration ranges of the calibration and unknown solutions must fall within the multimeter measurement range. So, verify whether the multimeters are suitable to be used, and then make any pertinent adjustments to the calibration solutions.
- Another consideration to keep in mind: if the multimeters measure resistance through the application of direct current, electrode polarization (as explained in Figures 1 and 2) may hinder the ability to record measurements.
- If not having students prepare their own solutions of sodium chloride in accordance with the worksheet, make enough of each solution so that each group can take about 100 ml for its own measurements (about 2 liters for each calibration solution).
- Prepare a cup of another aqueous solution of NaCl to serve as the unknown concentration solution for all teams to measure.
- Prepare a cup of pickle juice for all teams to measure.
- Hand out the supplies, solutions, worksheets and a BASIC Stamp conductivity meter to each group.
- Make sure that the switch for the BASIC Stamp is in the "0" (off) position.
With the Students
- Review the following scientific information with the class:
For electrons to pass through a liquid environment, charge carriers must be present in the solution to accept electrons from one electrode and deposit them at the other, thus completing the circuit. These charge carriers, known as ions, transition from a neutral state in solid form, to charged entities in solution. For example, sodium chloride exists as a neutral crystal of sodium and chloride atoms in its solid form. When placed in water, the atoms of sodium and chloride begin to move away from each other as they interact with water molecules. In doing so, they no longer maintain a neutral state. Rather, they become charged ions: the chlorine atoms adopt negative charges and the sodium atoms take on positive charges. It is the presence of these ions that allows electrons to be transferred from a solid conductor, such as metal wire, to an electrolytic solution.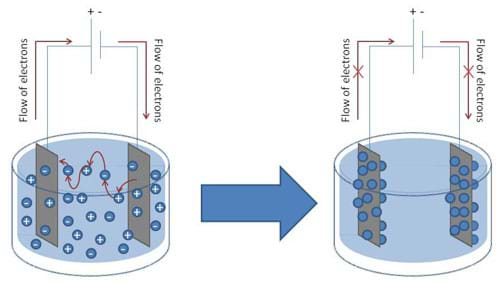
However, when measuring electrical signals in a liquid environment, certain precautions must be adhered to. In order for electrons to travel from a solid conductor (the conductivity probe in this case) to ions in solution, a chemical reaction must take place at the surface of each probe. The ions that are present in solution are mobile and move about accepting electrons from one probe and depositing them on the other, thus allowing a current to pass through the solution, completing the circuit. However, as the current continues to flow in the circuit in one direction, over time, a depletion of ions in solution occurs, as well as an accumulation of ions at the probe surfaces, which inhibits the flow of electrons. Thus, the surfaces of the probes become polarized, leading to a diminished current flowing through the circuit (see Figure 1).
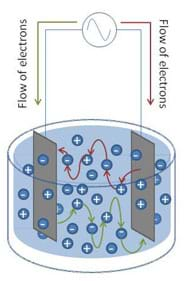
To overcome this phenomenon, an alternating signal is used to drive the current in opposing directions through the conductivity circuit (see Figure 2). The BASIC Stamp conductivity meter uses an integrated circuit called the 555 timer to accomplish this.
- Turn on the BASIC Stamp conductivity meter by moving the three-position switch to the "1" position. When the five LEDs blink, it is ready to be used. If the LEDs do not turn on, press the reset button.
- Have teams prepare the calibration solutions of sodium chloride in accordance with the worksheet.
- Starting with the calibration of lowest concentration, as indicated on the worksheet, immerse the conductivity probe (the two screws) up to about the blue cap (see Figure 3). Record on the worksheet the number of LEDs that light up. Wash the conductivity probe, by immersing it in a cup of water, seeing the LEDs blink. Then immerse it in the next calibration solution and take a reading. Repeat this process for each calibration solution (from lowest to highest concentration), recording the results. Tips: Make sure that the depth at which the conductivity probe is immersed is the same every time, and that the probes are thoroughly rinsed between measurements.
- Once a complete table of conductivity readings for various NaCl concentrations has been compiled, plot these data points using the grid on the worksheet.
- Using a ruler, draw a best fit line that is equidistant to all points on the graph. This graph serves as your conductivity calibration curve.
- Determine the concentration of an unknown aqueous solution of NaCl provided by the teacher.
- Pour three different colors/flavors of Gatorade (or any unknown salt solution that is within the calibration range of the meter) into cups to about the same level as the water during the conductivity testing. Immerse the conductivity probe (to the same depth), and measure its conductivity.
- Using the conductivity reading(s) (LED lights), refer to the conductivity calibration curve created earlier to estimate the equivalent concentration of NaCl that is contained in Gatorade. Record your estimate(s) in the data table on the worksheet.
- Determine the concentration of the sample of pickle juice provided by the teacher.
- Have students clean up the lab equipment and work spaces.
- Have students individually complete the worksheet questions and hand in the worksheets.
- Lead a concluding class discussion to compare results and conclusions.
Vocabulary/Definitions
BASIC Stamp: A single-board computer that runs the Parallax PBASIC language interpreter in its microcontroller. The developer's code is stored in an EEPROM, which can also be used for data storage. The PBASIC language has easy-to-use commands for basic I/O, such as turning devices on or off, interfacing with sensors, etc. More advanced commands enable the BASIC Stamp module to interface with other integrated circuits, communicate with each other, and operate in networks. The BASIC Stamp microcontroller has prospered in hobby, lower-volume engineering projects and education due to its ease of use and a wide support base of free application resources. See parallax.com.
capacitor: A device for accumulating and holding a charge of electricity, consisting of two equally charged conducting surfaces having opposite signs and separated by a dielectric (a non-conducting substance).
conductivity: A measure of how well materials permit the passage of electricity.
electrolyte: A conducting medium in which the flow of current is accompanied by the movement of matter in the form of ions.
electron: An elementary particle that is a fundamental constituent of matter, having a negative charge of 1.602 × 10^-19 coulombs, a mass of 9.108 × 10^-31 kilograms, and spin of 1/2, and existing independently or as the component outside the nucleus of an atom.
ion: An electrically charged atom or group of atoms formed by the loss or gain of one or more electrons.
resistor: A device designed to introduce resistance to the flow of current into an electric circuit.
Assessment
Activity Embedded Assessment
Worksheet: Have students use the worksheet as they conduct the lab. Evaluate students on the collection of data for the calibration curves. All curves should be linear with positive slopes. Review their data, graphs and answers to gauge their comprehension of the material.
Analysis: Expect students to be able to deduce the equivalent concentration of NaCl contained in the unknown solution(s). Students should make the connection that increases in ion concentration result in greater solution conductivity.
Activity Extensions
Ask students to research and propose possible uses of conductivity measurements in our society. Example answers: water purification (for example, desalination—converting salt water into drinking water) and battery technology (materials with enhanced conductivities).
Subscribe
Get the inside scoop on all things TeachEngineering such as new site features, curriculum updates, video releases, and more by signing up for our newsletter!References
Dictionary.com. Dictionary.com LLC. Accessed August 2, 2009. (Source of some vocabulary definitions, with some adaption)
Parallax.com. Parallax Inc. Accessed August 2, 2009. http://parallax.com (manufacturer of microcontroller development tools and small single-board computers, including the BASIC Stamp microcontroller and free downloadable software program used in this activity) http://www.parallax.com/Support/DownloadsPress/tabid/477/Default.aspx
Copyright
© 2009 by Regents of the University of Colorado; original © 2009 Polytechnic Institute of New York UniversityContributors
Keeshan Williams; Jill Fonda; Vikram KapilaSupporting Program
AMPS GK-12 Program, Polytechnic Institute of New York UniversityAcknowledgements
This activity was developed by the Applying Mechatronics to Promote Science (AMPS) Program funded by National Science Foundation GK-12 grant no. 0741714. However, these contents do not necessarily represent the policies of the NSF, and you should not assume endorsement by the federal government.
Last modified: February 25, 2020





User Comments & Tips