Quick Look
Grade Level: 6 (5-6)
Time Required: 3 hours
(can be split into different days)
Expendable Cost/Group: US $2.00
Group Size: 3
Activity Dependency: None
NGSS Performance Expectations:

| MS-ESS3-5 |

Summary
Students observe teacher-led demonstrations, and build and evaluate simple models to understand the greenhouse effect and the role of increased greenhouse gas concentration in global warming.Engineering Connection
To address global warming, engineers of all disciplines must understand the greenhouse effect and its causes, and then creatively design new technologies to reduce the production of greenhouse gases. Some engineers examine the types of chemicals released in the manufacturing process, and re-design new ways of production or methods to remove harmful chemicals before factory emissions are released into the atmosphere. Others re-design engines to make more efficient the fuel burning process and/or reduce chemical emissions.
Learning Objectives
After this activity, students should be able to:
- Explain that human activities increase carbon dioxide concentrations (air pollution).
- Explain that carbon dioxide gas is a greenhouse gas whose increased concentration in the atmosphere contributes to global warming.
- Build, test and evaluate different models of the greenhouse effect and global warming.
- Explain why engineers need to know the amount of carbon dioxide in the atmosphere.
Educational Standards
Each TeachEngineering lesson or activity is correlated to one or more K-12 science,
technology, engineering or math (STEM) educational standards.
All 100,000+ K-12 STEM standards covered in TeachEngineering are collected, maintained and packaged by the Achievement Standards Network (ASN),
a project of D2L (www.achievementstandards.org).
In the ASN, standards are hierarchically structured: first by source; e.g., by state; within source by type; e.g., science or mathematics;
within type by subtype, then by grade, etc.
Each TeachEngineering lesson or activity is correlated to one or more K-12 science, technology, engineering or math (STEM) educational standards.
All 100,000+ K-12 STEM standards covered in TeachEngineering are collected, maintained and packaged by the Achievement Standards Network (ASN), a project of D2L (www.achievementstandards.org).
In the ASN, standards are hierarchically structured: first by source; e.g., by state; within source by type; e.g., science or mathematics; within type by subtype, then by grade, etc.
NGSS: Next Generation Science Standards - Science
| NGSS Performance Expectation | ||
|---|---|---|
|
MS-ESS3-5. Ask questions to clarify evidence of the factors that have caused the rise in global temperatures over the past century. (Grades 6 - 8) Do you agree with this alignment? |
||
| Click to view other curriculum aligned to this Performance Expectation | ||
| This activity focuses on the following Three Dimensional Learning aspects of NGSS: | ||
| Science & Engineering Practices | Disciplinary Core Ideas | Crosscutting Concepts |
| Ask questions to identify and clarify evidence of an argument. Alignment agreement: | Human activities, such as the release of greenhouse gases from burning fossil fuels, are major factors in the current rise in Earth's mean surface temperature (global warming). Reducing the level of climate change and reducing human vulnerability to whatever climate changes do occur depend on the understanding of climate science, engineering capabilities, and other kinds of knowledge, such as understanding of human behavior and on applying that knowledge wisely in decisions and activities. Alignment agreement: | Stability might be disturbed either by sudden events or gradual changes that accumulate over time. Alignment agreement: |
Common Core State Standards - Math
-
Use appropriate tools strategically.
(Grades
K -
12)
More Details
Do you agree with this alignment?
-
Represent real world and mathematical problems by graphing points in the first quadrant of the coordinate plane, and interpret coordinate values of points in the context of the situation.
(Grade
5)
More Details
Do you agree with this alignment?
International Technology and Engineering Educators Association - Technology
-
Students will develop an understanding of the effects of technology on the environment.
(Grades
K -
12)
More Details
Do you agree with this alignment?
-
Analyze how different technological systems often interact with economic, environmental, and social systems.
(Grades
6 -
8)
More Details
Do you agree with this alignment?
State Standards
Colorado - Math
-
Appropriate measurement tools, units, and systems are used to measure different attributes of objects and time.
(Grade
4)
More Details
Do you agree with this alignment?
-
Graph points on the coordinate plane to solve real-world and mathematical problems.
(Grade
5)
More Details
Do you agree with this alignment?
Colorado - Science
-
Interpret and analyze data about changes in environmental conditions – such as climate change – and populations that support a claim describing why a specific population might be increasing or decreasing
(Grade
6)
More Details
Do you agree with this alignment?
Materials List
Class Demo 1: Carbon Dioxide Extinguishes a Flame
- narrow-necked bottle with a narrow opening, such as a glass soda bottle
- vinegar
- baking soda
- matches
- candle
- plastic drinking straw
- Hot Stuff! Activity 1 Worksheets, one per student; use this worksheet for Class Demo 1, 2, 3, and Student Activity 1
Class Demo 2: Creating and Testing for Carbon Dioxide
- narrow-necked bottle with a narrow opening, such as a glass soda bottle
- 50 ml vinegar
- 45 grams baking soda
- small, round balloon
- plastic drinking straw
- 50 ml BTB; solution = 6 drops per 1/3 cup water; BTB is bromothymol blue (BTB), a dye used as an acid-base indicator solution; available at chemistry equipment stores, well-equipped hardware stores and online chemistry equipment supply companies such as Fischer Scientific International
- clear beaker, large enough to hold 50 ml of BTB solution
- Hot Stuff! Activity 1 Worksheets, one per student; use this worksheet for Class Demo 1, 2, 3, and Student Activity 1
Student Activity 1: Testing for Carbon Dioxide from Our Own Breath
Each student needs:
- small, round balloon
- plastic drinking straw
- small cup of ~50 ml of BTB solution; solution = 6 drops per 1/3 cup water; paper Dixie cups or small beakers are suitable
- Hot Stuff! Activity 1 Worksheet, use this worksheet for Class Demo 1, 2, 3, and Student Activity 1
Class Demo 3: Testing Ice
- 2 medium-size beakers, each half full of BTB solution; solution = 6 drops per 1/3 cup water
- 1-2 small pieces of ice (cubes)
- 1-2 small pieces of dry ice
- Hot Stuff! Activity 1 Worksheets, one per student; use this worksheet for Class Demo 1, 2, 3, and Student Activity 1
Student Activity 2: Team Investigations
Each group needs:
- Hot Stuff! Activity 2 Investigation Worksheet and Instructions; provide each group with the specific instruction sheet for the investigation that the group is conducting
- masking tape and pen
- graph paper, 1 sheet per group
- supplies for the specific investigation the group is conducting (see below).
- Investigation #1 supplies:
- 2 identical, large plastic jars
- 2 outdoor thermometers (must fit in jar)
- 25 g baking soda
- 1 cup vinegar
- plastic wrap (2 large squares to fit over jar openings)
- 2 rubber bands
- Investigation #2 supplies:
- 2 identical, large jars
- 2 outdoor thermometers (must fit in jar, below rim)
- hot water
- hot mitt
- plastic wrap (1 large square to fit over jar opening)
- rubber band
- Investigation #3 supplies:
- 2 identical, large jars
- 2 outdoor thermometers (must fit in jar, below rim)
- cold water chilled with ice cubes
- plastic wrap (1 large square to fit over jar opening)
- rubber band
- Investigation #4 supplies:
- 2 identical, large jars
- 2 outdoor thermometers (must fit in jar, below rim)
- 2 cups of garden soil
- plastic wrap (1 large square to fit over jar opening)
- rubber band
Worksheets and Attachments
Visit [www.teachengineering.org/activities/view/cub_air_lesson07_activity1] to print or download.Introduction/Motivation
Global warming is the increase in the Earth's average atmospheric temperature over a long period of time, generally due to increased levels of greenhouse gases caused by human activities. Scientists believe that even a 2-3ºF (0.6-1.1°C) increase in the average temperature of the Earth could trigger disasters.
This rise in average global temperature is caused by human activities, particularly the burning of fossil fuels. The amount of carbon dioxide entering the atmosphere increases when fossil fuels are burned and the excess carbon dioxide cannot be used by plants (especially since we are eliminating them, too). The excess carbon dioxide in the atmosphere absorbs heat from the sun and keeps it near the surface of the Earth, which raises the Earth's temperature. The concentration of carbon dioxide in the atmosphere has doubled in the last 100 years and scientists expect it to double in the next 100 years as well.
Scientists predict that these changes will trigger disaster. For example, a major shift in weather patterns could cause droughts, tropical storms and increase temperatures that would make some currently habitable areas of the Earth become uninhabitable. It is also speculated that melting polar ice caps could cause a rise in sea levels and, in turn, flood low-lying areas, such as coastal cities like New York City and San Francisco. Can you imagine if those high-population cities started to flood? The melting of the icecaps could also dilute marine saline concentrations, threatening marine life.
While most scientists believe that the greenhouse effect will gradually warm the Earth's climate, some scientists predict that as the temperature rises, more water will evaporate from the oceans, resulting in more clouds. This increase in clouds could block out sunlight, causing an overall decrease in the Earth's average temperature.
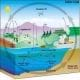
Forests have been called the "lungs of the Earth" because animals inhale oxygen and exhale carbon dioxide in the process of breathing, and plants take in carbon dioxide and give off oxygen in the process of photosynthesis (see The Carbon Cycle Diagram). Every year, more than 78 million acres of tropical forest are cut and burned to clear land for farming and ranching. According to the World Resources Institute, global loss of tropical forests contributed about 4.8 billion tonnes of carbon dioxide per year.
Environmental engineers are concerned about photosynthesis because plants help clean the air of harmful carbon dioxide gas, replacing it with oxygen. With the decreasing numbers of trees in the world, the air is not being cleaned as well. Additionally, the carbon dioxide levels continue to increase due to increasing numbers of automobiles and industrial pollution. Environmental engineers are continually challenged to find methods to reduce carbon dioxide emissions from industry and cars, and find ways to clean our polluted air.
Procedure
Background
This activity is composed of three teacher-led class demonstrations and two student-conducted activities. Students use the Hot Stuff! Activity 1 Worksheet for Class Demos 1, 2 and 3, and Student Activity 1. Students use the Hot Stuff! Activity 2 Investigation Worksheet and Instructions for Student Activity 2.
Bromothymol blue (BTB) changes from dark blue to light blue, green or yellow, depending on the concentration of carbon dioxide present.
Dry ice is solid carbon dioxide at -110 ºF.
Before the Activity
- Prepare the BTB solution: 6 drops BTB per 1/3 cup water.
- Gather materials and make copies of the worksheets.
- Make arrangements to transport ice and dry ice, and heat the hot water.
With the Students
Class Demo 1: Carbon Dioxide Extinguishes a Flame
- Explain the steps of the demonstration to the students. Ask them to make predictions about what will happen and record them on their Hot Stuff! Activity 1 Worksheets.

- Light the candle.
- Mix vinegar and baking soda in a bottle. This works best if you put the vinegar in the bottle first and then add the baking soda.
- Tilt the bottle slightly over a burning candle so that the gas gently flows out and extinguishes the candle. It may help to use a straw from the bottle to direct the CO2 towards the flame, see Figure 1. Do not tilt the bottle so much that the liquid runs out and extinguishes the flame.
- Ask students to record their observations on their worksheets.
- Ask the students to explain why the flame went out. Discuss each theory.
- Repeat the demonstration, explaining what is happening at each step. (Explanation: CO2 pushes away the O2 needed by the candle to keep burning.) Explain to students that they will create this same CO2 gas for another experiment.
Demo 2: Creating and Testing for Carbon Dioxide
- Explain the steps of the demonstration to the students. Ask them to make predictions about what will happen and record their predictions on their Hot Stuff! Activity 1 Worksheets.

- Pour 50 ml of vinegar into a narrow-necked bottle.
- Place 45 grams of baking soda in a balloon.
- Secure the rim of the balloon over the mouth of the bottle without releasing any of the baking soda into the vinegar.
- Tip the balloon so that the baking soda falls into the vinegar.
- Observe the inflation of the balloon (see Figure 2).
- Carefully twist the balloon shut so that the gas does not escape.
- Insert a straw into the balloon and squeeze the opening closed with your fingers. A little carbon dioxide might escape, but try to keep most of it in the balloon.
- Pour 50 ml of the BTB solution into a clear beaker.
- Explain to the students that this BTB solution is an acid-base indicator. (They should remember about these from Air Pollution unit, Lesson 6.) What do they think it will show? (Answer: BTB indicates the presence of carbon dioxide [an acid] with a color change; turning from blue to green or yellow.)
- Place the opposite end of the straw into the solution.
- Slowly allow the carbon dioxide to be released into the BTB solution.
- Have students observe what happen and record it on their worksheets. (Explanation: The BTB solutions turns from blue to green or yellow.)
Student Activity 1: Testing for Carbon Dioxide from Our Own Breath
- Explain the steps of the activity to the students. Ask them to make predictions about what will happen and record them on their Hot Stuff! Activity 1 Worksheets.
- Ask students to name the gas that animals exhale. (Hopefully, they know that it is CO2.)
- Distribute a small balloon, a straw, and a small cup of BTB solution to each student.
- Ask the students to predict if the concentration of CO2 that they exhale will be greater or less than the concentration from the vinegar and baking soda.
- Repeat the steps in Class Demo 2, picking up from step #8 as described above, except this time ask the students to blow up their balloons.
- Observe and record results on their worksheets. (Explanation: The BTB turns from blue to green or yellow.) What happens? How is using CO2 from their breath different from Demo 2?
Class Demo 3: Testing Ice
- Explain the steps of the demonstration to the students. Ask them to make predictions about what will happen and record them on their Hot Stuff! Activity 1 Worksheets.
- Fill two medium-sized beakers half-full of BTB solution.
- Ask the students to predict what will happen when you place a small piece of ice into a beaker.
- Place the ice into the beaker. (Nothing should happen.)
- Ask students to predict what will happen when you place a small piece of dry ice into a beaker.
- Place the dry ice (solid CO2) into the beaker.
- Ask students to record their observations on their worksheets. (Answer: BTB changes color, from blue to green or yellow, due to the presence of CO2.)
- Can students explain the difference in reactions? (Explanation: The BTB turns from blue to green or yellow in the presence of CO2. Regular ice is frozen water. Dry ice is frozen CO2.)
Student Activity 2: Team Investigations
- Divide the class into investigation teams of three students each.
- Assign each group to one of the four different investigations in the attached Hot Stuff Activity 2 Investigation Worksheet and Instructions. Have at least two groups working on each investigation.
- Distribute the appropriate instructions to each group.
- Ask each group to carefully read through their instructions and make their initial predictions about what will happen in their investigation.
- Have students gather their supplies, set up and begin. Investigation recaps and explanations:
Investigation #1
CO2 is released in the reaction (just like in the demos). This models an increase in greenhouse gas. While the jars heat up at about the same rate, the one with CO2 cools down much more slowly.
Investigation #2
This models how CO2 and water vapor in the atmosphere affect the rate at which the Earth loses heat (the plastic wrap acts as the CO2 and water vapor during the experiment).
Investigation #3
This is similar to Investigation #2. This models how CO2 and water vapor in the atmosphere affect the rate at which the Earth gains and loses heat (the plastic wrap acts as the CO2 and water vapor during the experiment).
Investigation #4
This models how the temperature of the actual Earth changes (as opposed to water in investigations #2 and #3). The plastic wrap acts as the greenhouse gases.
- Give students time to create graphs and think about their results.
- Suggest that each team make a presentation to the class. They could start with a brief outline for their presentation. Require each team member to participate in the presentation.
- After all the presentations are given, conduct a class discussion. Ask the students which model they think is the best representation of the greenhouse effect? Global warming? How would they improve the models?
Assessment
Pre-Activity Assessment
Predictions: Using the attached worksheets, have students make and record predictions about what will happen.
Activity Embedded Assessment
Observations: Have students observe what happens during the activity and record it on their worksheets.
Post-Activity Assessment
Conference Presentations: Have students prepare and give oral presentations on their investigations. Often engineers are asked to present their research to a group of their peers or interested persons in a way that is understandable and clearly expresses the conclusions of their research. Have students pretend to be engineers who are presenting their recent findings about the effects of carbon dioxide at an EPA (U.S. Environmental Protection Agency) conference. Ask the students how the model they investigated is the best representation of the greenhouse effect? Global warming? How would they improve the models?
Discussion Questions: Ask students to summarize what they have learned in this activity. Do this as an informal discussion or a written homework assignment.
Safety Issues
- Be sure to have a fire extinguisher nearby since you are working with a flame.
- To prevent accidental ingestion of BTB, have students blow up balloons and use a straw (just as for candle example) to bubble their CO2 into the BTB solution. As a safer alternative, use phenolthalene in water with a few drops of ammonia in it; it will not show the range of concentrations as well as the BTB, but it acts as an acid indicator when the CO2 is present.
Troubleshooting Tips
A glass soda bottle is ideal for attaching the balloon since its neck and opening are narrow.
To save some time conducting Student Activity 2, once the students measure a constant temperature for 5-6 minutes, have them move on to the next part of their investigation.
Plan on 60 minutes to conduct the three class demonstrations and Student Activity 1. Plan on another 60 minutes for Student Activity 2. Allow a final 60 minutes for student presentation of investigation results and a class discussion.
Activity Extensions
Use spreadsheets and computers to record data and generate graphs.
Encourage students to implement some of their ideas for improving the greenhouse effect/global warming models.
Research how much the Earth's average temperature has changed in the last 100-200 years. Make a chart using this information.
Have students visit a local greenhouse and record temperatures during the day (or obtain the data from personnel willing to help).
Get students to think about how they could test for CO2 levels in the classroom. What about CO2 produced by cars?
The amount of carbon dioxide in the air at the end of 2019 was 410 parts per million by volume (ppmv). This means that for every million molecules of gases in the air, 410 of them were carbon dioxide. This is equal to 0.041 of every 100 molecules. Ask students to write a paragraph or two describing what this means. You can also have them create a visual aid, such as 100 pennies, showing that just a small fraction of one penny would represent the amount of CO2.
Use global warming statistics information to make graphs and look for trends. Where are we headed? Is the planet's temperature change slowing, speeding up or staying the same?
Activity Scaling
- For grades 3-4, pick one of the investigations in Student Activity 2 and have all the teams conduct the same one.
- For grades 1-2 (younger children), conduct only the demonstrations (not the two student activities), and discuss as a class why CO2 can be harmful to humans and the Earth.
Subscribe
Get the inside scoop on all things TeachEngineering such as new site features, curriculum updates, video releases, and more by signing up for our newsletter!More Curriculum Like This
Students observe teacher-led demonstrations, and build and evaluate simple models to understand the greenhouse effect, the role of increased greenhouse gas concentration in global warming, and the implications of global warming for engineers, themselves and the Earth. In an associated literacy activ...
Students are introduced to the concept of energy cycles by learning about the carbon cycle. They learn how carbon atoms travel through the geological (ancient) carbon cycle and the biological/physical carbon cycle.
Students are presented with examples of the types of problems that environmental engineers solve, specifically focusing on air and land quality issues.
Students are introduced to the concepts of air pollution, air quality, and climate change. The three lesson parts (including the associated activities) focus on the prerequisites for understanding air pollution. First, students use M&M® candies to create pie graphs that express their understanding o...
References
Environmental Issues. Teacher Created Materials, 1994. Online at Teacher Created Resources. http://www.teachercreated.com/
Rain Forest – Extended Thematic Unit. Teacher Created Materials, 1995. Online at Teacher Created Resources. http://www.buyteachercreated.com/estore/product/0674
Science Plus – Technology and Society (Level Green). Holt, Rinehart and Winston Inc., 1997.
Williams, Jack. "Understanding Greenhouse Gases." Published November 7, 2000. Updated July 23, 2003. USA Today. July 22, 2004. http://www.usatoday.com/weather/climate/wco2.htm
Copyright
© 2004 by Regents of the University of ColoradoContributors
Amy Kolenbrander; Daria Kotys-Schwartz; Janet Yowell; Natalie Mach; Malinda Schaefer Zarske; Denise W. CarlsonSupporting Program
Integrated Teaching and Learning Program, College of Engineering, University of Colorado BoulderAcknowledgements
The contents of this digital library curriculum were developed under grants from the Fund for the Improvement of Postsecondary Education (FIPSE), U.S. Department of Education and National Science Foundation (GK-12 grant no. 0338326). However, these contents do not necessarily represent the policies of the Department of Education or National Science Foundation, and you should not assume endorsement by the federal government.
Last modified: September 6, 2020







User Comments & Tips