Quick Look
Grade Level: 10 (9-12)
Time Required: 2 hours 45 minutes
3 class blocks: ~35-45 min. on Day 1, ~35 min. on Day 2, ~90 min. on Day 3, plus two extension activities
Expendable Cost/Group: US $4.33 This activity also uses many non-expendable (reusable) items see the Materials List.
Group Size: 3
Activity Dependency: None
Subject Areas: Chemistry, Physics, Science and Technology
NGSS Performance Expectations:

| HS-ETS1-2 |
| HS-ETS1-3 |
| HS-PS4-5 |
Summary
Students gain first-hand experience with the steps of the scientific method as well as the overarching engineering design process as they conduct lab research with the aim to create a bioplastic with certain properties. Students learn about the light mechanism that causes ultraviolet bead color change, observe the effect of different light waves on a phosphorescence powder, and see the connection between florescence, phosphorescence and wavelength. Students compose hypotheses and determine experimental procedure details, as teams engineer variations on a bioplastic solid embedded with phosphorescence powder. The objective is to make a structurally sound bioplastic without reducing its glowing properties from the powder embedded within its matrix. Groups conduct qualitative and quantitative analyses of their engineered plastics, then recap and communicate their experiment conclusions in the form of a poster, slides and verbal presentation. As an extension, teams make their own testing apparatuses. As a further extension, they combine all the group results to determine which bioplastic matrix best achieves the desired properties and then “manufacture” the optimum bioplastic into glowing toy figurine end products! Many handouts, instructions, photos and rubrics are provided.Engineering Connection
Solar cells and solar panels are becoming common on rooftops, parking lots, solar farms, light-up street signs, moving gates, calculators, sidewalk night lights, and recharging devices. Inorganic solar cells, the most common cells used in solar panels, are considered efficient because they absorb a significant amount of energy from the sun. Yet, they are unable to turn all of the sun’s electromagnetic spectrum into usable energy; they are unable to convert most of the infrared and ultraviolet light. Inorganic solar cells are also expensive, large and stiff.
Newer technology organic solar cells are cost effective, flexible and compactable, which means they are even more useful for a wide variety of applications. However, organic solar cells are less efficient than inorganic cells because they absorb even less energy from sunlight than inorganic solar cells. So engineers are researching and experimenting with new manufacturing methods to improve the efficiency of organic solar cells.
Since solar cells are composed of coated layers with each layer responsible for absorbing certain light wavelengths, researchers are focused on synthesizing new layers to incorporate into solar cells that can absorb light wavelengths that current solar cells cannot. The design objective is to increase the energy efficiency of organic solar cells while keeping the cost effectiveness and mechanical properties that make organic solar cells so desirable.
In this activity, students are involved in a design challenge that is similar to this solar cell design challenge—to engineer a bioplastic that exhibits specific physical characteristics. In their lab research, groups isolate and experiment with varying different independent variables in the composition of a phosphorescent bioplastic, aiming to find a chemical composition that best meets the desired material properties.
Learning Objectives
After this activity, students should be able to:
- Investigate the effects of various light (UV, infrared, visible) on phosphorescence.
- Define phosphorescence, florescence and electromagnetic spectrum.
- Distinguish between independent and dependent variables.
- Create a phosphorescence bioplastic.
- Test the various concentrations of the components found in bioplastic (corn starch, water, vinegar, glycerin) to determine if a change in concentration inhibits or strengthens its phosphorescence.
- Perform qualitative and quantitative analysis.
Educational Standards
Each TeachEngineering lesson or activity is correlated to one or more K-12 science,
technology, engineering or math (STEM) educational standards.
All 100,000+ K-12 STEM standards covered in TeachEngineering are collected, maintained and packaged by the Achievement Standards Network (ASN),
a project of D2L (www.achievementstandards.org).
In the ASN, standards are hierarchically structured: first by source; e.g., by state; within source by type; e.g., science or mathematics;
within type by subtype, then by grade, etc.
Each TeachEngineering lesson or activity is correlated to one or more K-12 science, technology, engineering or math (STEM) educational standards.
All 100,000+ K-12 STEM standards covered in TeachEngineering are collected, maintained and packaged by the Achievement Standards Network (ASN), a project of D2L (www.achievementstandards.org).
In the ASN, standards are hierarchically structured: first by source; e.g., by state; within source by type; e.g., science or mathematics; within type by subtype, then by grade, etc.
NGSS: Next Generation Science Standards - Science
| NGSS Performance Expectation | ||
|---|---|---|
|
HS-ETS1-2. Design a solution to a complex real-world problem by breaking it down into smaller, more manageable problems that can be solved through engineering. (Grades 9 - 12) Do you agree with this alignment? |
||
| Click to view other curriculum aligned to this Performance Expectation | ||
| This activity focuses on the following Three Dimensional Learning aspects of NGSS: | ||
| Science & Engineering Practices | Disciplinary Core Ideas | Crosscutting Concepts |
| Design a solution to a complex real-world problem, based on scientific knowledge, student-generated sources of evidence, prioritized criteria, and tradeoff considerations. Alignment agreement: | Criteria may need to be broken down into simpler ones that can be approached systematically, and decisions about the priority of certain criteria over others (trade-offs) may be needed. Alignment agreement: | |
| NGSS Performance Expectation | ||
|---|---|---|
|
HS-ETS1-3. Evaluate a solution to a complex real-world problem based on prioritized criteria and trade-offs that account for a range of constraints, including cost, safety, reliability, and aesthetics, as well as possible social, cultural, and environmental impacts. (Grades 9 - 12) Do you agree with this alignment? |
||
| Click to view other curriculum aligned to this Performance Expectation | ||
| This activity focuses on the following Three Dimensional Learning aspects of NGSS: | ||
| Science & Engineering Practices | Disciplinary Core Ideas | Crosscutting Concepts |
| Evaluate a solution to a complex real-world problem, based on scientific knowledge, student-generated sources of evidence, prioritized criteria, and tradeoff considerations. Alignment agreement: | When evaluating solutions it is important to take into account a range of constraints including cost, safety, reliability and aesthetics and to consider social, cultural and environmental impacts. Alignment agreement: | New technologies can have deep impacts on society and the environment, including some that were not anticipated. Analysis of costs and benefits is a critical aspect of decisions about technology. Alignment agreement: |
| NGSS Performance Expectation | ||
|---|---|---|
|
HS-PS4-5. Communicate technical information about how some technological devices use the principles of wave behavior and wave interactions with matter to transmit and capture information and energy. (Grades 9 - 12) Do you agree with this alignment? |
||
| Click to view other curriculum aligned to this Performance Expectation | ||
| This activity focuses on the following Three Dimensional Learning aspects of NGSS: | ||
| Science & Engineering Practices | Disciplinary Core Ideas | Crosscutting Concepts |
| Communicate technical information or ideas (e.g. about phenomena and/or the process of development and the design and performance of a proposed process or system) in multiple formats (including orally, graphically, textually, and mathematically). Alignment agreement: | Solar cells are human-made devices that likewise capture the sun's energy and produce electrical energy. Alignment agreement: Information can be digitized (e.g., a picture stored as the values of an array of pixels); in this form, it can be stored reliably in computer memory and sent over long distances as a series of wave pulses.Alignment agreement: Photoelectric materials emit electrons when they absorb light of a high-enough frequency.Alignment agreement: Multiple technologies based on the understanding of waves and their interactions with matter are part of everyday experiences in the modern world (e.g., medical imaging, communications, scanners) and in scientific research. They are essential tools for producing, transmitting, and capturing signals and for storing and interpreting the information contained in them.Alignment agreement: | Systems can be designed to cause a desired effect. Alignment agreement: Science and engineering complement each other in the cycle known as research and development (R&D).Alignment agreement: Modern civilization depends on major technological systems.Alignment agreement: |
Common Core State Standards - Math
-
Summarize, represent, and interpret data on a single count or measurement variable
(Grades
9 -
12)
More Details
Do you agree with this alignment?
International Technology and Engineering Educators Association - Technology
-
Students will develop an understanding of the role of troubleshooting, research and development, invention and innovation, and experimentation in problem solving.
(Grades
K -
12)
More Details
Do you agree with this alignment?
-
Students will develop an understanding of the attributes of design.
(Grades
K -
12)
More Details
Do you agree with this alignment?
-
Students will develop an understanding of engineering design.
(Grades
K -
12)
More Details
Do you agree with this alignment?
-
Students will develop abilities to apply the design process.
(Grades
K -
12)
More Details
Do you agree with this alignment?
-
Students will develop an understanding of the relationships among technologies and the connections between technology and other fields of study.
(Grades
K -
12)
More Details
Do you agree with this alignment?
State Standards
Mississippi - Math
-
Summarize, represent, and interpret data on a single count or measurement variable
(Grades
9 -
12)
More Details
Do you agree with this alignment?
Mississippi - Science
-
Apply inquiry-based and problem-solving processes and skills to scientific investigations.
(Grades
9 -
12)
More Details
Do you agree with this alignment?
-
Demonstrate an understanding of general properties and characteristics of waves.
(Grades
9 -
12)
More Details
Do you agree with this alignment?
Materials List
For the intro activity:
- UV beads, 4 per student; such as from a 2-ounce package of (~250) ultraviolet detecting beads for $7 at Educational Innovations Inc.
- string, 6-inches per student; OR pipe cleaner, one per student
- LED UV flashlight, also known as a “black light,” such as 1 nine-LED UV flashlight for $6 each at Art n’ Glow or 5 for $12 at Amazon
- LED flashlight, such as 4 nine-LED mini aluminum flashlights for $20 at Amazon or 1 for $1 at Wal-Mart
- extra AA or AAA batteries for flashlights, if not included with purchased flashlights
- heat lamp, such as a 250-watt incandescent infrared red heat lamp light bulb with brass base for $9 at Wal-Mart
- (optional) cameras or cell phones, to take photos of beads
- a small amount of phosphorescent powder; such as from a 1-ounce pouch at Art n’ Glow, for the intro light station demonstration
- (optional) black film canister, in which to place a small amount of phosphorescent power, such as from a 20-pack for $11 at Amazon
- Intro Activity Handout, one per person
- Electromagnetic Spectrum Handout, one per person
- access to a computer and projector, to show the Intro Activity Presentation, a PowerPoint® file
- (optional) example fluorescent and phosphorescent items to show students, such as highlighter ink (fluorescent), glow-in-the-dark stickers and toys (phosphorescent) and/or black light posters
For the research, hypothesis and experimental step, each group needs:
- Problem, Research and Hypothesis Handout, one per person
- access to a computer with Internet, to conduct reactant research
- Lab Procedure for Samples Handout, the four sheets of this 16-page file that pertain to the group’s assigned reactant (if four students in team, each student gets one of the four sheets)
- hot plate
- 4 petri dishes, such as 20 for $6.25 at Flinn Scientific
- Sample Size Template; this sheet includes 20 sample templates (little square shapes to cut out) and each group needs four, so print as many of this sheet as needed
- permanent marker, to label petri dishes
- aluminum foil, to line some petri dishes
- 2 silicone spatulas, such as a heat-resistant silicone spreader for $1.88 each at Wal-Mart
- timer or stopwatch; cell phones work well for this task
- 1,000-ml beaker
- 100-ml graduated cylinder
- 2 x 10-ml graduated cylinder, for vinegar and glycerin
- 2 x 250-ml beaker, so one can cool between making samples
- electronic balance
- weight paper and metal spatulas, for measuring corn starch and phosphorescent powder
- 2 disposable pipettes, for measuring vinegar and glycerin
- corn starch, ~500 grams for entire class (total reactant amounts depend on group experiment designs)
- vinegar, ~200 ml for entire class
- glycerin, ~200 ml for entire class, such as from a 6 fluid ounce (177 ml) bottle of Humco USP emollient demulcent for $3.88 at Wal-Mart pharmacy
- water, tap water is fine
- phosphorescent (daytime white/glow) powder; one 1-ounce pouch of daytime white, white glow powder for $15 at Art n’ Glow
For quantitative analysis, each group needs:
- box cutter or scalpel, for sample prep **monitor and stress careful use of these sharp tools**
- 4 petri dishes, such as 20 for $6.25 at Flinn Scientific
- scotch tape
- permanent marker, to label petri dishes
- Quantitative Data Table Handout, one per person
- Qualitative Data Table Handout, one per person
For one teacher-prepared quantitative testing apparatus to share with the class, as described in the Testing Apparatus Construction Step-by-Step Instructions (a PowerPoint® file):
- ring stand with 2 clamps
- cardboard box, 29.3 x 20.3 x 12 cm; this box size available at Wal-Mart
- masking tape
- box cutter
- UV light, the same flashlight used for the intro activity
- light sensor probe, such as the LS-BTA light sensor for $55 at Vernier; see alternative suggestion below
- LabQuest, a standalone interface used to collect sensor data with built-in graphing and analysis application; such as the LabQuest2 for $329 at Vernier; see alternative suggestion below
- access to computer to run LabQuest software
- Student-Created Testing Apparatuses & Procedure Write-Ups/Slides Grading Rubric, one per group
To share with the entire class:
- an adequate supply of safety glasses, heat gloves and lab aprons
Equipment alternative: If cost or availability of the light probe and LabQuest are problematic, students can perform qualitative analysis (instead of quantitative analysis using the light probe and LabQuest) of the samples’ phosphorescent glow by charging all samples with a large UV light (such as this 18-inch fluorescent lamp, a black light, for $43 at Flinn Scientific) and then ordering/ranking them by their glow (see Figure 2).
For making (optional extension) student-created testing apparatuses for quantitative analysis, each team needs a cardboard box (can be of various sizes such as package boxes, cereal boxes and/or bulk candy boxes (like those that hold multiple candy bars at store check-out lines; have students bring from home during the week before the activity) and the same rest of the materials as listed above for the teacher-prepared testing apparatus.
For the poster/presentation conclusion, each group needs:
- poster board or large dry erase board, 60 x 80 cm
- markers
- meter stick
- Conclusion Presentation Grading Rubric, one per group
- Conclusion Poster Layout Handout, one per group
- access to a computer/projector with PowerPoint® software
For (optional) second activity extension to analyze combined group data and make glowing toys:
- Class Data Table Handout, one per person
- silicone molds, such as a set of 7 Star Wars characters silicone ice cube trays for $15 at Amazon
- (optional) spray lubricant, for the silicone molds before bioplastic added
Worksheets and Attachments
Visit [www.teachengineering.org/activities/view/usm-2091-lab-research-design-phosphorescent-materials] to print or download.Pre-Req Knowledge
Students need a basic understanding of the steps of the scientific method. As necessary, review this concept using the Scientific Method Example Foldable or see Table 1.
Introduction/Motivation
(Intro activity) Today, engineers and scientists are continually researching ways to create new and improved products that we use every day to help solve daily problems and improve our quality of life. To do this, scientists are guided by the steps of the scientific method while engineers are guided by the steps of the engineering design process. But they often work together and mingle together the processes.
What’s the difference? Scientists investigate what is; they discover new knowledge about our world by peering into the unknown. Engineers create what has not been; they make things that have never existed before. (Source: Joe Bordogna, former deputy director, National Science Foundation)
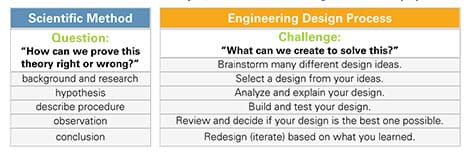
The scientific method and engineering design process are essential practices used in a wide variety of disciplines. Both are a series of logical steps that help scientists and engineers answer questions, develop theories and explanations, and/or find solutions to problems (see Table 1). These practices have helped to add to the body of knowledge that forms our understanding of our world and how it works. These approaches are the basis for all research that is carried out by university faculty researchers, undergraduate and graduate students, and professional investigators today.
Many of you are familiar with the steps of the scientific method and the steps of the engineering design process. Both can involve lab experimentation. We use the scientific method to make sure we conduct systematic experiments to collect and analyze data, and when we use the engineering process, our ultimate goal is to create something new that meets specified objectives. Sometimes these approaches come together when we conduct lab research with the purpose to design chemical compounds with certain properties (criteria and constraints) for use as end products.
(Bring up the slides and use them as you go through the following information with the class, reviewing the big idea of the intro bead activity and how it relates it to the concepts of florescence, phosphorescence and the electromagnetic spectrum. As necessary, use additional diagrams and/or demos to explain the electromagnetic spectrum, electromagnetic waves, wavelength and frequency. Then divide the class into groups of three or four students each.)
(slide 2) Ask students to share with the class their qualitative findings about the beads. Example answer: The beads changed from white to a color after being exposed to sunlight.)
(slides 3-4) Here is a diagram of the electromagnetic spectrum. The electromagnetic spectrum contains waves ranging from x-rays to radio waves. The wave types differ from each other based on their wavelengths, which is the distance from one crest of a wave to the next. X-rays have short wavelengths and radio waves have long wavelengths. Waves also differ based on frequency, which is the number of waves passing a certain point in one second; x-rays have a high frequency and radio waves have a short frequency.
Sunlight is composed of different wavelengths of light: ultraviolet (UV), visible light, and infrared. UV light has short wavelengths and infrared light has long wavelengths; visible lights falls between them. You may have heard of UV light from sunscreen commercials since this light is the type of radiation that tans our skin. (If desired, discuss in more depth.) Let’s test the beads under different types of light (different wavelengths).
(slide 5) For testing, these three light stations provide us with three wavelengths: UV light (a black light flashlight]) visible light (a LED flashlight), and infrared light (a heat lamp). (Hold some beads under each station. Have students make observations and then conclude that the beads change color under the UV light.) The “black light” that causes the beads to change color is an artificial source of UV light.
(Introduce the following problem for students to solve using the scientific method. Recap: Students act as engineers to manipulate a reaction scheme in order to develop a bioplastic embedded with phosphorescent powder that is structurally sound but still gives off a high phosphorescent glow.)
Today, you are going to engineer a product similar to the beads. You will create a bioplastic—a biodegradable plastic created from biological substances and not petroleum—that is embedded with glowing powder. Currently engineers are working on ways to create materials that are cost effective, efficient and exhibit the desired mechanical properties that enable the material to have various applications. In this black film canister, is the glowing powder you will use, but first we need to do some research with the glowing powder.
(slide 6) First, let’s determine what type of light activates the glowing powder. (Have students hold the film canister of powder under each of the three light stations and observe under which light it glows/reacts the best. Answer: The powder glows best under the UV light.) As you can see, like the beads, the powder also glows when placed under the black light.
(slide 7) Next, let’s determine if the glowing powder is florescent or phosphorescent. Do you know these terms? And what is the difference between these terms? What are examples of each? (Refer to the slide for definitions, as well as the Vocabulary/Definitions section. If possible, show students some fluorescent and phosphorescent examples; see the Materials List. As examples, students may be familiar with fluorescent highlighter ink and phosphorescent glowing ceiling star stickers.
(slide 8) Is the powder florescent or phosphorescent? (See if students have been paying attention.) That’s right, the powder is phosphorescent, meaning it glows when exposed to ultraviolet radiation and continues to glow after the source of UV light is removed. A fluorescent powder would stop glowing once the black light was turned off.
(Continue on as described in the Procedure section, using slides 9-10.)
Procedure
Before the Activity
- Gather materials and make copies of the various handouts listed in the Materials List and in the Procedure section. Note that the 16-page Lab Procedure for Samples Handout includes the sheets needed by four teams; each team gets the four sheets to guide varying the amount of its assigned reactant—corn starch, water, vinegar and glycerin; so make enough copies for however many teams in your class.
- Set up group lab stations with the needed materials.
- Prepare a quantitative testing apparatus for the class to use, as described in the Testing Apparatus Construction Step-by-Step Instructions, for use on Day 3. This uses a UV flashlight, light probe, LabQuest and computer.
- (optional extension) Decide if you want to have students create and use their own testing apparatuses, and plan accordingly. Doing this adds an additional class block to the overall activity. See detailed instructions in the Activity Extension section. Doing this also provides the added opportunity for students to test their samples using both the team-created testing apparatus as well as the teacher-created testing apparatus.
- Follow the instructions in the Sample Preparation Presentation to create one example prepared petri dish (a petri dish with a paper square taped to its outside base and a labeled lid) to show students.
- Be ready with a computer and projector to show the class some PowerPoint® files: Intro Activity Presentation, Lab Procedure for Standard/Control Sample Preparation, Sample Preparation Presentation, Testing Apparatus Construction Step-by-Step Instructions, Examples of Bioplastic Toys and Silicone Molds (extension activity).
- For the Day 1 intro activity, use the items listed in the Materials List to set up three light stations—for UV light (black light flashlight), visible light (LED flashlight) and infrared light (heat lamp).
- Note that samples created on Day 2 of the activity may take up to 48 hours to dry and cure before they are used on Day 3 of the activity.
- (optional extension: class shared data > product manufacture) Decide if you want to have groups combine all their results so teams can examine the class data in order to identify a sample that has the desired properties in order to manufacture bioplastic glowing toy figurines—which they proceed to create in silicone molds. This adds more time to the overall activity. See more details in the Activity Extension section. Doing this shows students how what is learned through scientific and engineering research is applied to the creation of an end product.
With the Students: Before Day 1—Informal Bead Research
- The day before the activity, at the end of class, give students a few minutes to each make a bracelet of beads using UV beads and string (or pipe cleaner). Do not tell them that they are UV beads!
- Assign students the following task: “Wear these beads today and tomorrow, and observe any changes. Tomorrow you will share your findings with the class.” If students have access to cameras or phones with cameras, suggest that they take “selfies” of themselves with the beads if they notice any changes.
With the Students: Day 1—Intro Activity, Problem, Hypothesis & Experiment Steps
- For the intro activity, begin by presenting the Introduction/Motivation content, which includes use of the three light stations, Electromagnetic Spectrum Handout, Intro Activity Handout, and the 11-slide Intro Activity Presentation (slides 1-8) as an outline/supportive teaching tool. This includes students sharing their bead observations and using the three light stations, and the teacher dividing the class into research groups of three or four students each and introducing the problem/challenge.
- Direct students to complete the Problem Step and Hypothesis Step (described below), guided by a handout and previewed by using the rest of the slides (9-11) as an outline to walk students through the two steps.
- (slide 9) Problem Step: Hand out the Problem, Research and Hypothesis Handout. Inform students of the following research problem: Your challenge is to manipulate a reaction scheme that creates a bioplastic embedded with phosphorescent powder so that the bioplastic has structural integrity—it doesn’t fall apart—but still gives off a high phosphorescent glow. From an engineering point of view, these objectives are considered the criteria and constraints of the design challenge.
- Provide students with the following reaction scheme:
corn starch + water + vinegar + glycerin + phosphorescent powder > phosphorescent bioplastic
- Assign each group a reactant to manipulate: corn starch, water, vinegar or glycerin. (If more than four groups, assign two groups corn starch and/or two groups water.)
- (slide 10) Have each group research its reactant. Suggested research prompts:
- What is your reactant?
- How is it commonly used?
- Is it commonly used in baking? If so, why?
- What does structural integrity mean to you?
- List your prior knowledge about the reactant.
- (slide 11) Hypothesis Step: Using their research, have teams each develop a testable hypothesis statement. Hypotheses must include how the reactants are expected to contribute to the bioplastic’s structural integrity and phosphorescent glow. Make sure students are clear on what are the independent variable and dependent variables.
Example hypothesis: Increasing the corn starch concentration in the bioplastic reaction scheme will cause the bioplastic to be harder, increasing structural integrity, but will make it cloudy, causing a decrease in its phosphorescent glow.
- Experiment Step: Have all groups first each create a standard/control sample, guided by the Lab Procedure for Samples Handout (refer to sheet 1 of 4 for each group’s reactant) and/or teacher-directed using the 15-slide Lab Procedure for Standard/Control Sample Preparation. Below are the experimental steps:
- In a 1,000-ml beaker, add 10 grams of corn starch (using weigh paper and a balance).
- Using a 10-ml graduated cylinder, add 5 ml of vinegar to the same beaker.
- Using a 10-ml graduated cylinder, add 5 ml of glycerin to the same beaker.
- Using a 100-ml graduated cylinder, add 60 ml of water to the same beaker. USE YOUR MEASURED WATER TO GET THE GLYCERIN RESIDUE FROM THE GRADUATED CYLINDER IN THE PREVIOUS STEP.
- Using a silicone spatula, stir the mixture until the corn starch is dissolved and the mixture is thoroughly combined.
- Using a graduated cylinder, measure 60 ml of the mixture and dispense it into to a clean 250-ml beaker.
- Add 0.6 grams of the phosphorescence solid to the 60-ml mixture in the 250-ml beaker (from the PREVIOUS STEP).
- Using a clean silicone spatula, stir the mixture until the phosphorescence solid is completely dissolved throughout.
- Using a hot plate preheated to 400 °C, heat the mixture in the 250-ml beaker.
- USING THE SILICONE SPATULA, CONTINUOUSLY STIR THE MIXTURE WHILE HEATING.
- For 6 minutes and 30 seconds, continue to heat and stir the mixture until it becomes clear or becomes a viscous solid that is hard to stir. ** BE CONSISTANT WITH TIME FOR ALL YOUR SAMPLES **
- Using the silicone spatula, transfer the heated mixture into a small petri dish that is lined with aluminum foil and labeled with the sample name.
- Let the phosphorescence bioplastic dry and harden overnight.
With the Students: Day 2—Lab Design & Lab Work
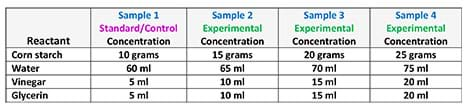
- Based on their standard/control and their research, have groups decide how they intend to alter the concentrations of their reactants in order to create three more samples for a total of four samples per team. See Table 2 for example sample concentrations for the four reactants.
IMPORTANT: In planning their experiments, direct the groups to consider the following:
- Going down in concentration from the standard/control concentrations is not possible with water because 60 ml of the mixture is needed in step 6 of the procedure, so encourage students to increase concentrations when making samples
- When determining sample concentrations, create a trend, such as going up by 5 ml for each vinegar sample.
- Have students move through the sample fabrication process (creating samples that vary the amounts of a specific reactant), and record their quantitative and qualitative material observations in the observation column of their procedure pages from the appropriate sheets of the Lab Procedure for Samples Handout.
- Direct the groups to set aside their samples in a place where they can dry and cure overnight; samples may take up to 48 hours to dry and cure.
With the Students: Day 3—Sample Prep & Q/Q Analysis
- In order to obtain consistent data, all experimental sample sizes need to be the same. So direct students to cut samples using the Sample Size Template and box cutters/scalpels. Alert students to be very careful when using the sharp tools—or else have the teacher do this part. ***Note: KEEP the EXTRA PIECES in the ORIGINAL LABELED PETRI DISHES for later QUALITATIVE ANALYSIS***
- Give each group a new set of four petri dishes.
- Have students tape one sample size template to the back bottom of a petri dishes (show students the teacher-created sample petri dish). They use this as one of their petri dishes AND as a template to prep all the rest of their petri dishes to receive bioplastic samples.
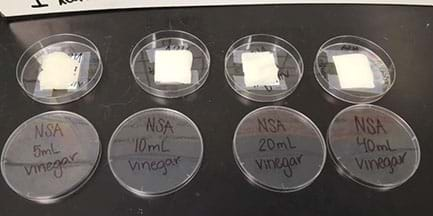
- Students label each petri dish with the original sample name from the beginning of the Experiment Step (see Figure 1).
- Students place the corresponding samples into their new testing petri dishes (refer to the Sample Preparation Presentation and Photos of Student Sample Examples).
- Students perform quantitative analysis with their prepared samples using the teacher-prepared testing apparatus (and/or their own team-created testing apparatuses, if they have done this optional activity extension). (Use the Quantitative Data Table Handout) Procedure for using the testing apparatus (also on the handout and slide 19 of the Testing Apparatus Construction Step-by-Step Instructions):
- Turn on the LabQuest and light probe.
- Set a timer for 2 minutes.
- Place a testing sample in place in the testing apparatus.
- Close the door (to shut out ambient light).
- Turn on the UV flashlight.
- After 2 minutes, turn off the UV light and quickly record the first 5 numbers from the light probe and LabQuest.
- Average the 5 readings to get 1 quantitative measurement of the bioplastic’s phosphorescent glow.
Alternative: If a light probe and LabQuest are not available for quantitative analysis, instead have students perform qualitative analysis of the samples’ phosphorescent glow by charging all samples with a large UV light and then ordering/ranking them by their glow (see Figure 2).

- Lastly, students use the extra pieces from the sample prep to perform qualitative analysis. Following the instructions on the Qualitative Data Table Handout, students test the sample pieces for transparency, hardness, flexibility, impact resistance, tension, and its ability to stick to a vertical surface (a wall).
With the Students: Day 3—Conclusion Poster & Presentation
Have groups each create a poster board presentation to outline the experiment, results and conclusions, as described in the Assessment section.
(optional extension) Class Shared Data > Product Manufacture
At this point, if desired, have groups combine all their results so teams can examine them in order to identify a sample that exhibits the optimum properties, which they proceed to create and manufacture as bioplastic glowing toy figurines made in silicone molds. See more details in the Activity Extension section. Doing this is an example of how results learned through scientific and engineering research is applied to the creation of an end product.
Vocabulary/Definitions
conclusion : The last step of the scientific method when scientists analyze their collected data in order to determine whether hypotheses are correct or not.
control group: In lab work, the test group that is treated “normally” to minimize the effects of variables other than the single independent variable and thus increase the reliability of the results. It does not contain the independent variable.
dependent variable: The results of the independent variable. It represents the output or outcome whose variation is being studied.
electromagnetic radiation: Waves of radiation that form when an electric field couples with a magnetic field.
electromagnetic spectrum: The entire range of wavelengths and frequencies of electromagnetic radiation. Examples include ultraviolet, infrared and visible light waves.
engineering design process: A series of steps that guide engineers in their quests to create solutions (new structures, products or systems) that meet the problem’s criteria and constraints.
experiment: The third step in the scientific method when scientists create a procedure and collect data in order to test hypotheses.
experimental group: In lab work, the test group that is treated differently; it contains the independent variable.
florescence: The emission of visible light by a substance that has absorbed UV radiation, and that ceases to glow when the radiation source stops.
hypothesis : The second step in the scientific method when scientists compose hypotheses, which are proposed, testable statements based on previous research/observations and prior knowledge.
independent variable: The variable being tested by the experimental research.
infrared light: Invisible light waves (electromagnetic radiation) given off by the sun with longer wavelengths than visible light. Most commonly produced by hot objects and can cause the temperature to rise.
phosphorescence: The emission of visible light by a substance that has absorbed UV radiation, and that continues to glow after the radiation source stops. Different than fluorescence, it’s absorbed radiation is re-emitted at a lower intensity (longer wavelength) for up to several hours after the original excitation.
problem : The first step in the scientific method when scientists state a research question and research background about it.
qualitative data: Data collected by using the human senses. Generally, these are observed properties that cannot be measured with numerical results.
quantitative data: Data collected by taking tangible measurements that have numerical characteristics.
scientific method: A series of steps that guide scientists to answer questions posed in the form of hypotheses. The steps are: problem > hypothesis > experiment > conclusion.
ultraviolet light: Light waves produced when an electric current is passed through ionized gases between two electrodes; also present in sunlight. Its wavelengths are shorter than visible light but longer than x-rays. Abbreviated as UV. Can be produced by specialized lights such as tanning lamps and black lights. Long-wavelength UV radiation plays a key part in photosynthesis, and causes skin tanning, chemical reactions and many substances to glow or fluoresce.
visible light: Light waves given off by the sun that human eyes can detect and are seen as different colors. Visible light wavelengths are shorter than infrared light but longer than UV light.
Assessment
Pre-Activity Assessment
Research and Hypothesis: After researching their reactants, have groups each compose a hypothesis based on what they think the effect their assigned reactant will have on the bioplastic’s structural integrity and phosphorescent glow—the design challenge objectives.
Activity Embedded Assessment
Experiment & Lab Design/Work: Have students document all of their observations, both quantitative and qualitative, on the appropriate sheets of the Lab Procedure for Samples Handout as they move through sample creation. Expect students to be active participants in sample creation, prep and testing.
Post-Activity Assessment
Concluding Poster and Presentation: Assign groups to each create a brief poster board presentation that they share with the class. Require the poster boards to outline the team’s experiment, results and conclusions. See the Photos of Student Poster Examples for past examples of student posters.
As guided by the Conclusion Poster Layout Handout, posters must include the following components: title, reaction scheme, independent variable, dependent variable, hypothesis, identified control group and explanation, identified experimental group(s) and explanation, quantitative results, and qualitative results.
With expectations provided by the Conclusion Presentation Grading Rubric, every student must play a role in the presentation and teams must include the following in their presentations:
- Present the poster.
- State the hypothesis.
- Identify the independent and dependent variables.
- Outline the experiment.
- Identify the control group and why it is the control group.
- Identify the experimental group(s) and why they are the experimental groups.
- Provide a table with the quantitative results.
- Provide a table with the qualitative results.
- Present their results conclusions.
During the final group presentations, ask students the Investigating Questions and grade each team using the rubric.
Investigating Questions
During the final group presentations, ask students the following questions:
- What were you testing? (Example answer: How the concentration of my reactant affects the phosphorescence and mechanical properties of my created bioplastic.)
- Why did you choose the experimental group reactant concentrations? (Example answer: The standard sample was created with 5 ml of glycerin, so we decided to increase our concentrations in increments of five: 10 ml, 15 ml, 20 ml.)
- If you could add a sample concentration, which would you add and why? (Example answer: If we could add a sample, we would do 13 ml of glycerin to see if it would support the trend seen with the other samples.)
- If you could repeat your experiment, what would you do differently? (Example answer: If we could repeat our experiment, we would be more careful to avoid any human error when collecting quantitative data with 10 ml of glycerin and 20 ml of glycerin samples because these do not follow the trend.)
- Which one of your samples was the most structurally sound and how do you know? (Example answer: According to our data, our 15 ml of glycerin sample was hard, flexible and impact resistant, making it the most structurally sound material.)
- Which one of your samples had the best phosphorescent glow and how do you know? (Example answer: According to our data, our 20 ml of glycerin sample gave the best phosphorescent glow of 13.66 lux, but we would like to test it again to confirm that number.)
- Which one of your samples meets the design challenge objectives best and why? (Example answer: We think that our 15 ml of glycerin sample is the best because it has a high phosphorescent glow 5.06 lux and structural integrity of hardness, flexibility and impact resistance.)
- If you could manufacture one of your samples, which one would you manufacture and why? (Example answer: We would manufacture our 15 ml of glycerin sample because of having a high phosphorescent glow and its hardness and flexibility characteristics, giving it more potential applications.)
Safety Issues
- Carefully monitor students when they are using the very sharp box cutters and/or scalpels.
- Use safety glasses, gloves and lab aprons.
- Wear heat gloves when using hot plates.
Activity Extensions
Student-Created Testing Apparatuses & Testing (adds another class block to the overall activity): Have groups each create their own testing apparatus made from cereal, packaging or candy boxes, box cutters and masking tape. Have teams design, write-out a procedure for constructing their testing apparatus and take pictures as they construct the testing apparatus. Require teams to incorporate their photos in PowerPoint® procedure write-ups that describe how they created their own testing apparatuses; see Four Student Examples of Testing Apparatuses and Procedure Write-Ups/Slides. Consider having teams test their samples using both the team-created and teacher-created testing apparatuses.
Have teams create their testing apparatuses using a UV flashlight and light sensor probe. Set up the UV flashlight and light probe with LabQuest on ring stands at a table so students can see this equipment. With expectations provided by the Student-Created Testing Apparatuses & Procedure Write-Ups/Slides Grading Rubric, advise students to consider the following:
- Make sure your samples are ONLY exposed to light you control.
- Make sure all your samples get the same amount of controlled direct UV light.
- Consider the light probe structure and dimensions.
- Use the metric system.
- Between sample tests, make sure minimal shifting of the testing apparatus and probe occurs.
When testing samples with their own testing apparatus, have students use the Quantitative Data Table Handout and follow the same procedures as described earlier (also on the handout and slide 19 of the Testing Apparatus Construction Step-by-Step Instructions):
- Turn on the LabQuest and light probe.
- Set a timer for 2 minutes.
- Place a testing sample in place in the testing apparatus.
- Close the door (to shut out ambient light).
- Turn on the UV flashlight.
- After 2 minutes, turn off the UV light and quickly record the first 5 numbers from the light probe and the LabQuest.
- Average the 5 readings to get 1 quantitative measurement of the bioplastic’s phosphorescent glow.
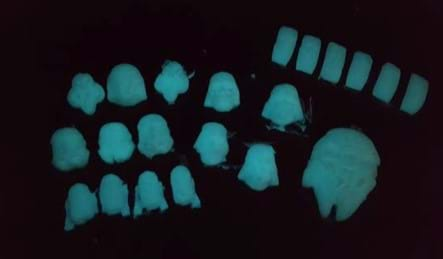
Class Shared Data > Product Manufacture (adds more time to the overall activity): Have all groups combine their results into the Class Data Table Handout. Then, looking at all the data, teams decide which sample has the properties they desire in order to manufacture a bioplastic into a glowing toy. Then each group fabricates that sample and makes a toy by pouring the bioplastic into a silicone mold sprayed with lubricant. For example, Star Wars character silicone molds have been used to make glowing toy figurines; see the Examples of Bioplastic Toys and Silicone Molds, a PowerPoint® file.
Subscribe
Get the inside scoop on all things TeachEngineering such as new site features, curriculum updates, video releases, and more by signing up for our newsletter!More Curriculum Like This

Students are introduced to the correct technical vocabulary for lighting, which is different than layperson's terms. They learn about lamp (light bulb) technology and how to identify the various types of lighting in their spaces. They are also introduced to lighting controls as a means for saving en...
References
Askari Mohammad Bagher. “Comparison of Organic Solar Cells and Inorganic Solar Cells.” International Journal of Renewable and Sustainable Energy. Vol. 3, No. 3, May 2014, pp. 53-58. doi: 10.11648/j.ijrse.20140303.12. http://www.sciencepublishinggroup.com/journal/paperinfo.aspx?journalid=169&doi=10.11648/j.ijrse.20140303.12
Miller, Joe; and Levine, Joe. Biology, Foundation Edition. Pearson Prentice Hall, 2010.
Wertheim, Jane. The Usborne Illustrated Dictionary of Physics. Usborne Books, 2011.
Copyright
© 2017 by Regents of the University of Colorado; original © 2016 University of Southern MississippiContributors
Jamie Sorrell, Sumrall High School, Sumrall, MS; Michael Hipp, Oak Grove High School, Hattiesburg, MSSupporting Program
Research Experience for Teachers Program, School of Polymers and High Performance Materials, University of Southern MississippiAcknowledgements
This activity was developed under the Research Experiences for Teachers (RET) in Engineering and Computer Science Site for Sustainable Polymer Engineering Research program in the University of Southern Mississippi’s School of Polymers and High Performance Materials, funded by National Science Foundation RET grant no. EEC 1406753. However, these contents do not necessarily represent the policies of the NSF, and you should not assume endorsement by the federal government.
Last modified: May 11, 2020





User Comments & Tips