Quick Look
Grade Level: 9 (8-9)
Time Required: 1 hour
Expendable Cost/Group: US $3.00 This activity also uses some non-expendable (reusable) items such as LED flashlights and computers; see the Materials List.
Group Size: 4
Activity Dependency: None
Subject Areas: Physical Science
NGSS Performance Expectations:

| HS-PS4-5 |

Summary
Students take what they know about materials, optical properties and electrons to the next level—to see how semiconductors can be used to augment light. First, they learn how light-emitting diodes (LEDs) work, which helps them to think critically about a real-world problem they are asked to solve later in the activity as if they are practicing engineers. The challenge: To design an improved LED headlight that lights the roadway without distracting oncoming drivers and passengers with the harsh, bright white light seen in many cars today. Students research the problem via an online video, article and interactive simulation, learning all about quantum dots. Then teams use small LED flashlights and pieces of red, blue, yellow and green acetate to independently experiment to come up with a model that has the potential to improve the measured visual quality of bright white LED light—their solutions to the headlight challenge.Engineering Connection
We live in a world in which improvement and optimization are continual goals. Engineers are instrumental in evolving the latest cutting-edge technology and are currently working to improve lighting properties by using quantum dots. Quantum dots are semiconductor nanocrystals that glow when stimulated by an external source such as ultraviolet (UV) light. In this activity, students see how quantum dots are used to increase the lighting quality of LEDs. Because of their ability to enhance color brightness and definition, quantum dots are increasingly used in modern day technology. For example, TV manufacturers employ quantum dots to make TVs brighter and improve color quality. Quantum dots eliminate the filtering process used in LED TVs, which lowers the energy use while producing brighter colors. Engineers also utilize quantum dot technology in personal electronics, such as to increase battery life in cell phones.
Learning Objectives
After this activity, students should be able to:
- Apply their prior knowledge about the periodic table, semi-conductors and wave properties to explain how light-emitting diodes produce light.
- Demonstrate how colors can be used to dim the lighting of LEDs.
- Create a visual representation of various color combinations colors to maximize LED lighting quality.
Educational Standards
Each TeachEngineering lesson or activity is correlated to one or more K-12 science,
technology, engineering or math (STEM) educational standards.
All 100,000+ K-12 STEM standards covered in TeachEngineering are collected, maintained and packaged by the Achievement Standards Network (ASN),
a project of D2L (www.achievementstandards.org).
In the ASN, standards are hierarchically structured: first by source; e.g., by state; within source by type; e.g., science or mathematics;
within type by subtype, then by grade, etc.
Each TeachEngineering lesson or activity is correlated to one or more K-12 science, technology, engineering or math (STEM) educational standards.
All 100,000+ K-12 STEM standards covered in TeachEngineering are collected, maintained and packaged by the Achievement Standards Network (ASN), a project of D2L (www.achievementstandards.org).
In the ASN, standards are hierarchically structured: first by source; e.g., by state; within source by type; e.g., science or mathematics; within type by subtype, then by grade, etc.
NGSS: Next Generation Science Standards - Science
| NGSS Performance Expectation | ||
|---|---|---|
|
HS-PS4-5. Communicate technical information about how some technological devices use the principles of wave behavior and wave interactions with matter to transmit and capture information and energy. (Grades 9 - 12) Do you agree with this alignment? |
||
| Click to view other curriculum aligned to this Performance Expectation | ||
| This activity focuses on the following Three Dimensional Learning aspects of NGSS: | ||
| Science & Engineering Practices | Disciplinary Core Ideas | Crosscutting Concepts |
| Communicate technical information or ideas (e.g. about phenomena and/or the process of development and the design and performance of a proposed process or system) in multiple formats (including orally, graphically, textually, and mathematically). Alignment agreement: | Information can be digitized (e.g., a picture stored as the values of an array of pixels); in this form, it can be stored reliably in computer memory and sent over long distances as a series of wave pulses. Alignment agreement: Photoelectric materials emit electrons when they absorb light of a high-enough frequency.Alignment agreement: Multiple technologies based on the understanding of waves and their interactions with matter are part of everyday experiences in the modern world (e.g., medical imaging, communications, scanners) and in scientific research. They are essential tools for producing, transmitting, and capturing signals and for storing and interpreting the information contained in them.Alignment agreement: | Systems can be designed to cause a desired effect. Alignment agreement: Modern civilization depends on major technological systems.Alignment agreement: Investigating or designing new systems or structures requires a detailed examination of the properties of different materials, the structures of different components, and connections of components to reveal its function and/or solve a problem.Alignment agreement: |
International Technology and Engineering Educators Association - Technology
-
Students will develop an understanding of the relationships among technologies and the connections between technology and other fields of study.
(Grades
K -
12)
More Details
Do you agree with this alignment?
State Standards
Georgia - Science
-
Identify the characteristics of electromagnetic and mechanical waves.
(Grade
8)
More Details
Do you agree with this alignment?
-
Students will analyze the properties and applications of waves.
(Grades
9 -
12)
More Details
Do you agree with this alignment?
-
Students will communicate scientific investigations and information clearly.
(Grades
9 -
12)
More Details
Do you agree with this alignment?
Materials List

- 1 LED flashlight, any size although ideal if they are all the same across groups so as to be able to compare results across groups; available at Amazon or home improvement stores like Home Depot., such as a three-pack for ~$10 at http://www.homedepot.com/p/Defiant-80-Lumens-LED-Flashlight-3-Pack-HD15FL04-3/206525460
- red, blue, yellow and green acetate film cut into rectangular strips sized to cover the flashlight outer lens, one of each color per group; available at various vendors such as www.utrechtart.com
- (optional) white paper, 2 sheets per student, to serve as journals
- pencils
- ruler
- black butcher paper, 6 feet; alternatively; black construction paper
- computer, on which to look at online videos/articles and run the PHET Color Vision interactive simulation at https://phet.colorado.edu/en/simulation/legacy/color-vision, which requires Java to run, which is available at https://java.com/en/download/mac_download.jsp
To share with the entire class:
- (optional) computer with Internet access and projector, to show the class an online video; alternatively: have students use their individual team computers
Pre-Req Knowledge
Background knowledge of the organization of the periodic table, the properties of metals and non-metals, and the properties of electrons.
Introduction/Motivation
Bright lights can be great or a nuisance—it depends on the context. For example, imagine it’s nighttime and you are riding in a car down a narrow, two-way road around a hill. Heading towards you is a car with LED headlights. The LED lights provide great visibility for the driver, but are almost blinding to the driver in your car who is faced with harsh, bright, white lights! Those super bright lights make it difficult to see, and can even be unnerving for passengers. How might you work as engineers to help solve this problem for your parents and future drivers (which one day will be you!)?

Today, you will take on the role of engineers who work for a company that designs LED lights for vehicles. Your company has called on you to design a new LED headlight that lights the way without distracting drivers with the harsh bright white light that many cars have today.
Many bright white light LEDs contain a large amount of blue light, which is appears harsh to human eyes. Natural sunlight has more green and red light and appears less harsh to human eyes, but LEDs are different. The majority of white LEDs are made by using a blue LED and converting a small portion of the blue light to yellow using a yellow phosphor, which results in a harsh white light. Though this method of creating white light is effective, it is not very efficient.
A question that engineers and researchers are currently exploring is how to maximize the efficiency of LED lights while controlling luminescence—or the emission of light—and color quality. The use of color is a viable method to maximize the lighting properties of LEDs. If we are able to mix in some additional colors into existing white light LEDs, then we may be able to improve the color quality as it appears to human eyes. You will use this technique today as you work to design new LED headlights.
Procedure
Background
Quantum dots are semiconductor nanocrystals that glow when stimulated by an external source such as ultraviolet (UV) light. Since quantum dots can be designed to meet specific size qualifications, they are ideal for improving the color quality of white light LEDs by mixing new colors into the color spectrum. Thus, it is possible to tune the spectrum to improve visual color quality by adding green and red colors similar to natural sunlight. The following videos explain quantum dot technology in liquid crystal display (LCD) screens and how quantum dot filters work:
Quantum Dot TVs: Explained! by Marques Brownlee (3:13 minutes): https://www.youtube.com/watch?v=dIfLmM0YkXM
Better Lighting with Quantum Dots: A Brilliant Solution by The Economist (7:05 minutes): https://www.youtube.com/watch?v=VjznErmcLnU
Additional quantum dot applications include improving visual displays, such as LCDs. Remixing white LED light with red, green and yellow enhances the colors in smartphones, TVs and tablets. Currently, LCDs use white light that has been changed via a number of filters, which reduces the efficiency and limits the color quality of displays. Lighting and display efficiency can be improved by working with more efficient quantum dots and primary colors that are used to make a wide variety of colors.
As educators, we can challenge students to extend their knowledge of how they perceive color through the exploration of quantum dots. Humans perceive color when white light is absorbed, reflected, refracted, scattered or diffracted by matter. For example, when sunlight shines on a blue car, light at the wavelength for blue is reflected off the car and we see blue. The interference of light by matter is often emphasized in classrooms while light emission and conversion often receives less attention. During this activity, students combine their knowledge about materials, semiconductors, electrons and optical properties to understand how light-emitting diodes produce light. Students also demonstrate an understanding of how quantum dots can be used to enhance the color of white light LED technology.
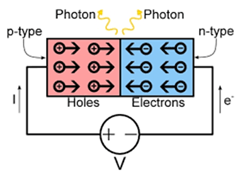
LEDs are tiny light bulbs that emit light by the movement of electrons in a semiconductor. When some of the electrons combine with holes, the process emits light that is equal to the bandgap of the semiconductor. The quantum dots are smaller semiconductor materials that absorb light, which causes electrons to be excited from the valence band to the conduction band. When these excited electrons relax, they give off light of a different color. By changing the size of the quantum dot, the energy gap in quantum dots can be artificially changed in order to vary the color of the light emitted from the quantum dot. Now, by combining LEDs (the light emission source) with quantum dots (the light conversion material), we can shift some of the color emitted by the LED and quantum dot assembly. By choosing specific quantum dots, engineers can explore how to expand the color spectrum emitted by white light LEDs by using quantum dots to fill in spectral gaps. In addition to enhancing the color emitted, quantum dots can augment the harsh LED light. In this activity, students apply their knowledge about wave properties to determine which color combination to mix in order to enhance LED light quality.
Before the Activity
- Gather supplies. Cut the colored acetate film into rectangles that fit over the flashlight outer lens.
- Be ready to show the class an online video (1:17 minutes) called, How Does an LED Work? at https://www.youtube.com/watch?v=BH9LI973H8w.
- Make sure student group computers are ready for them to open the PHET Color Vision interactive simulation that is available at https://phet.colorado.edu/en/simulation/legacy/color-vision.
With the Students: Engage
- Conduct the informal pre-activity assessment, as described in the Assessment section.
- Present to the class the Introduction/Motivation content, which introduces the engineering challenge: To design a new LED headlight that illuminates the roadway ahead without distracting oncoming drivers with a harsh bright white light.
- To pique interest in the topic and conduct some research about the problem, show students the online How Does an LED Work? video that describes what the N-type and P-type semiconductors are experiencing inside LED lights.
- Either as a class discussion or individual written responses, ask students to answer the following questions based on what they learned from the video:
- What happens to the positive and negative charges once current is added? (Answer: Once current is added, negative charges move in one direction and positive charges move in the opposite direction. The free electrons combine with a hole that exists at a lower excitation state. The electrons lose energy to combine with the positive hole; as a result, a photon of light is given off.)
- What determines the frequency or color of light emitted? (Answer: The amount of energy released determines the frequency or color of light emitted.)
- If students are familiar with the ROYGBIV acronym for the visible light spectrum, tell them that higher energy corresponds with higher frequency and that energy increases from R to B. For instance, an LED that emits blue light releases more energy than an LED that emits red light.
With the Students: Explore
- Remind students of the engineering challenge with the following prompt: “You are engineers solving a real-world problem. Your company has called on you to design a new LED headlight that lights the roadway without distracting oncoming drivers with the harsh bright white light that many cars have today. You just learned about how LED lights work. Now you will observe how color is mixed using a simulation.”
- Inform students that the mixing of primary colors—red, blue and green—produces all the colors we see.
- Suggest that students think about mixing paint; for example, adding blue to red produces purple. Light works in much the same way.
- Divide the class into groups of four students each and have each team assemble at a computer.
- Direct students to open the PHET Color Vision interactive simulation at https://phet.colorado.edu/en/simulation/legacy/color-vision.
- Have students adjust the red, green and blue scale to create a number of colors. Then have them summarize in their journals their results from manipulating the colors.
- Ask students if they notice any trends or patterns. (Expect students to see that mixing all the colors produces white light and that by adjusting the level of colors that make it to the human eye, various colors are perceived.)
- Tell students to keep this in mind as they design a combination of added colors that reduces the harshness of the LED headlights. (Note: From the simulation, expect students to see how varying the intensity of primary colors creates the colors that we see.)
- If groups finish early, challenge them to record in their journals how to create specific colors such as red, orange, yellow, green, blue, indigo and violet.
- Remind students that you have discussed how different colors could be emitted based on the type of materials used in an LED. Then direct students to read about quantum dots at the Nanosys website at http://www.nanosysinc.com/what-we-do/quantum-dots. Ask them to write in their journals their answers to the following questions:
- What determines the electronic characteristics of quantum dots? (Answer: Their sizes and shapes.)
- How can we change the color of light using quantum dots? (Answer: Using different sizes of quantum dots changes the colors they emit.)
- If you wanted red light, would you use a larger or smaller quantum dot? Why? (Answer: We would use a larger quantum dot, because larger dots emit longer wavelengths, which means shorter frequencies, giving a color closer to red; see Figure 1. Note: The Figure 1 graph shows how quantum dots can emit a wide range of colors. When creating quantum dots, researchers alter the dot size to obtain specific colors.)

With the Students: Explain
Remind students that they saw how we perceive color in the PHET simulation, and they saw how LED lights work and that the quantum dot size affects the color emitted. Ask students:
- What can we vary within the LED to change the color of light emission? (Answer: Varying the level of photon energy released alters the frequency or color of light emitted. The materials used in the semiconductor can also influence the color emitted.)
With the Students: Evaluate
- Tell the students: “Up until now, you have been engaging in an important step of the engineering design process: researching the problem. Now, you are ready to apply what you have learned to work through the next steps of the engineering design process to solve the real-world problem posed earlier. Remember, you are engineers who work for a company that designs LED lights for cars. A manufacturing client wants to use LEDs in its headlights, but does not want oncoming drivers to be distracted by the harsh white light LEDs usually emit. As an engineer, your challenge is to design a method to reduce the blue light in the LED so that it is easier on the eyes of other drivers and passengers. You are given the following materials: colored acetate paper, white LED flashlight, ruler and black paper. Working in your teams of four engineers, determine which color combination dims the white LED light the best by measuring the light intensity (instructions below).”
- Direct students in the following procedure to measure the light intensity.
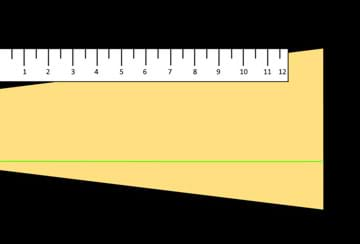
- Using black construction paper as a background, hold the flashlight against the background and measure the length of the brightness from the middle of the flashlight to the end of the brightness (see Figure 2).
- Add combinations of acetate films of your choice and measure the resulting brightness, being accurate in recording the combinations and length (brightness) in your journals. It is up to your team to decide which combination dims the bright LED light most effectively. (Hint: Consider overlapping the films to get the effect you want.)
- Teacher note: The colored acetate paper mimics the effect of quantum dot filters. Quantum dot filters causes color changes based on controlling the semiconductor size. Colored acetate paper changes the color of the LED due to filtration.
- To conclude, conduct a post-activity reflection as described in the Assessment section.
Vocabulary/Definitions
electron: An elementary charge that circulates the outer portion of an atom and carries a negative charge as current flows through a circuit.
hole: A positive elementary charge that flows opposite to electrons in a circuit.
light-emitting diode: A two-lead semiconductor with a p-n junction, designed to light up in a direct current (DC) circuit. Abbreviated as LED.
luminescence: The emission of light while not emitting heat.
quantum dot: Tiny, human-made crystals that can emit light across the visible spectrum.
semiconductor: A substance that has a conductivity between that of a metal and an insulator.
Assessment
Pre-Activity Assessment
Gauging Base Knowledge: As an informal warm-up and assessment, have students jot down the reasons humans see color. (Possible answers: White light from the sun gets reflected off surfaces at certain wavelengths, white lights travels through colored filters, etc.)
Activity Embedded Assessment
Discussion: During the activity, pique students’ interest in color and lighting. Ask them how theater lights are used to create certain moods. For example, a yellow gel placed on the white light fixture creates light that suggests a sunset feeling. This helps students see how color is used to augment light.
Recording Designs: During the activity, make sure students are recording in their journals their research, color combinations and measurements. Review their entries to gauge their comprehension of the topic and task.
Post-Activity Assessment
Reflection: After the activity, have students reflect on their designs, and compare and contrast the different models used to decrease the brightness of the light. Expect successful designs to be some combination of non-blue films to make the light less harsh, yet still strongly illuminating over distance.
Activity Extensions
If you have access to quantum dot filters, place them over LED lights to dim their brightness. You may want to review in advance the video listed in the Additional Multimedia Support section about how the technology works. Quantum dot filters can be purchased from QD Vision; company information is given in the video. Have students research the latest advancements made using quantum dot technology.
Additional Multimedia Support
QD Vision uses quantum dot filters to enhance the lighting properties of LED light bulbs. Teachers may want to preview the following video to see how colored acetate paper can mimic the effect of quantum dot filters. Better Lighting with Quantum Dots: A Brilliant Solution by The Economist (7:05 minutes) at https://www.youtube.com/watch?v=VjznErmcLnU.
The Color Vision PHET interactive simulation used in the activity is available to download at https://phet.colorado.edu/en/simulation/legacy/color-vision.
Subscribe
Get the inside scoop on all things TeachEngineering such as new site features, curriculum updates, video releases, and more by signing up for our newsletter!More Curriculum Like This

Students are introduced to the correct technical vocabulary for lighting, which is different than layperson's terms. They learn about lamp (light bulb) technology and how to identify the various types of lighting in their spaces. They are also introduced to lighting controls as a means for saving en...

Through three teacher-led demonstrations, students are shown samplers of real-world nanotechnology applications involving ferrofluids, quantum dots and gold nanoparticles. This nanomaterials engineering lesson introduces practical applications for nanotechnology and some scientific principles relate...
References
Facts about light-emitting diodes (LEDs). Science Facts, Science With Kids. Accessed June 2015. http://sciencewithkids.com/science-facts/facts-about-LEDs.html
Landolfi, Rob. What causes different colors in flames? Q/A, Ask Experts, Physics & Astronomy Online, PhysLink.com. Washington DC. Accessed June 2015. http://www.physlink.com/Education/AskExperts/ae569.cfm
Moynihan, Tim. What are quantum dots, and why do I want them in my TV? Published January 19, 2015. Gear, Wired. Accessed June 2015. http://www.wired.com/2015/01/primer-quantum-dot/
Quantum dot. Reference terms. ScienceDaily.com. Accessed June 2015. (Excerpted from Wikipedia article on same topic) http://www.sciencedaily.com/terms/quantum_dot.htm
What are quantum dots? What can we do with quantum dots? Nanosys, Inc., Milpitas, CA. Accessed June 2015. (Source of background information and student assigned reading) http://www.nanosysinc.com/what-we-do/quantum-dots
Copyright
© 2017 by Regents of the University of Colorado; original © 2015 Georgia Institute of TechnologyContributors
Bertina BanksSupporting Program
Partnerships for Research, Innovation and Multi-Scale Engineering (PRIME) RET, Georgia TechAcknowledgements
This activity was developed by the Partnerships for Research, Innovation and Multi-Scale Engineering (PRIME) Research Experience for Teachers (RET) Program at Georgia Institute of Technology, funded by National Science Foundation RET grant no. EEC 140718. However, these contents do not necessarily represent the policies of the NSF, and you should not assume endorsement by the federal government.
Special thanks to Jamila Cola and the PRIME/GIFT program for the opportunity to complete this work.
Last modified: September 3, 2021






User Comments & Tips